Search API
Following the discovery of Human Immunodeficiency Virus (HIV) as a causative agent of AIDS, multiple vaccine-candidate clinical trials have been conducted over the past 35 years.
According to the authors of a new study published in the journal Science, future HIV vaccine candidates may be more successful if they include additional doses or persist longer in the body to stimulate the immune system further.
These researchers wrote on December 14, 2023, that the potential of an HIV vaccine might be better judged by measuring how it affects CD8+ T-cell function and sensitivity in addition to just assessing the number of CD8+ T cells generated, which has been the usual practice.
These findings build on decades of research by the HIV-Specific Immunity Section of NIAID’s Laboratory of Immunoregulation to better understand the immune response to HIV.
The insights from this study may help guide future preventive and therapeutic HIV vaccine design and development and HIV immunotherapy approaches.
Mark Connors, M.D., chief of the HIV-Specific Immunity Section of NIAID’s Laboratory of Immunoregulation, is available to discuss this research via e-mail at [email protected].
As of December 23, 2023, there are no approved HIV vaccines.

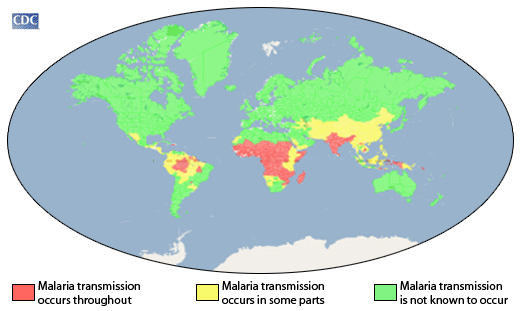
While malaria vaccines have become more available in Africa during 2023, they are not available in the Region of the Americas, where they are needed to protect children.
As disease-carrying mosquitos expand their range, tourists in vacation hot-spots such as the Republic of Costa Rica's breaches are left unprotected against this disease.
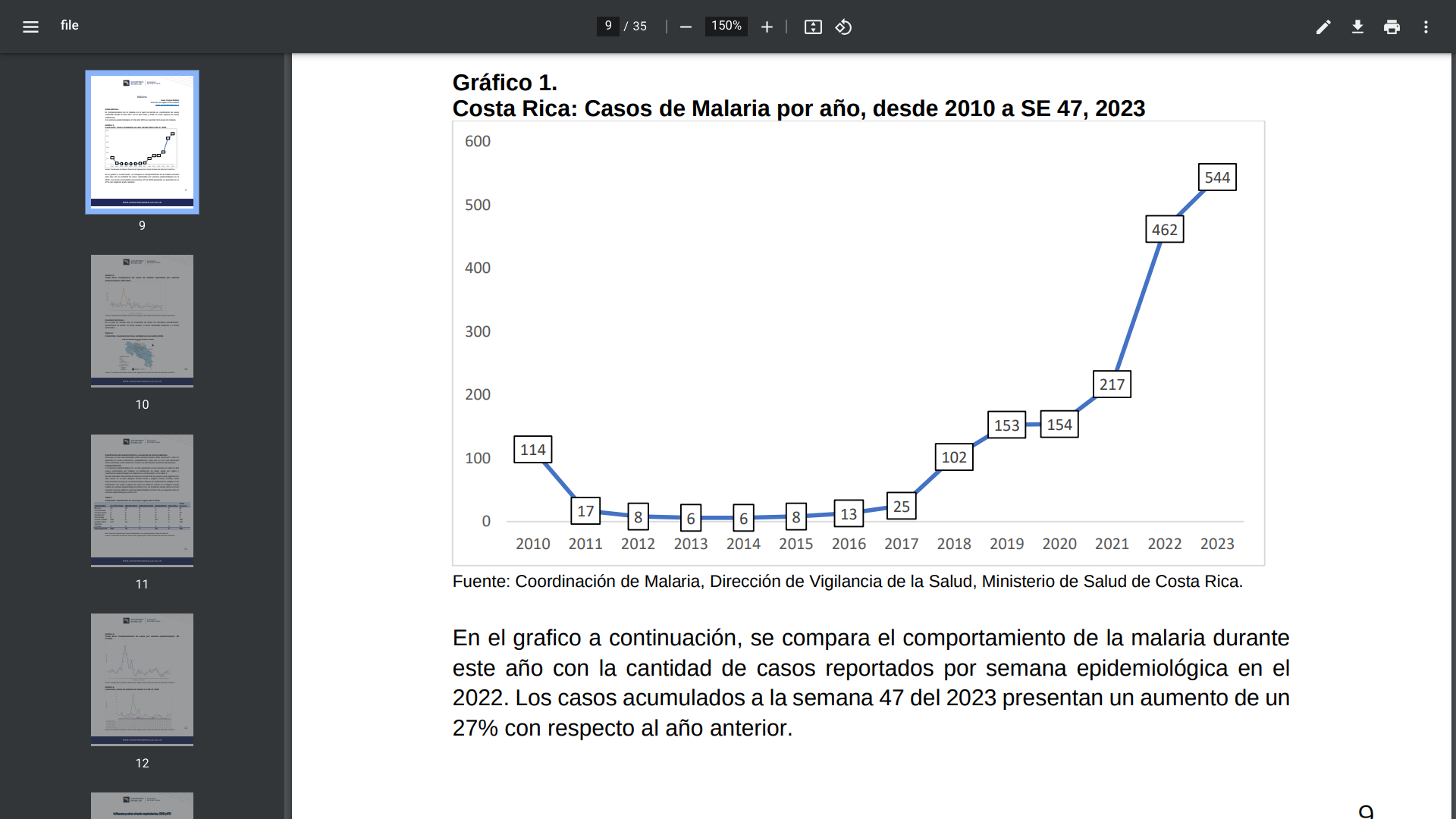
The Central American country of Costa Rica confirmed in December 2023 that there have been 544 malaria cases this year.
In 2022, Costa Rica's Health Ministry reported 406 locally-acquired malaria cases.
Globally, there were an estimated 249 million malaria cases and 426,000 deaths in 2022.
This year, the U.S. CDC recommends travelers visiting Costa Rica take prescription medicine to prevent malaria, but it can't suggest vaccinations yet.
As of October 2023, the World Health Organization (WHO) recommends two malaria vaccines for children: RTS,S/AS01, and R21/Matrix-M™.
The WHO expects these vaccines to have a positive public health impact.
And on December 21, 2023, WHO announced that it prequalified the R21/Matrix-M malaria vaccine. Prequalification status enables United Nations agencies to procure the vaccine for eligible countries.
Since most malaria cases in the United States are travel-related, the CDC may not expedite its approval for these new vaccines.
However, during 2023, local malaria cases have been confirmed in Florida, Maryland, and Texas.
Barinthus Biotherapeutics plc today announced a project with the Coalition for Epidemic Preparedness Innovations (CEPI) and the University of Oxford, aiming to fast-track the development of the VTP-500 vaccine candidate for the prevention of Middle East Respiratory Syndrome (MERS) Coronavirus (CoV).
This new project intends to take this MERS vaccine through Phase II clinical trials and, if successful, on into the development of an investigational ready reserve of 100,000 doses.
The three-way partnership has awarded up to $34.8 million to Barinthus Bio in addition to funds previously committed to the University of Oxford.
VTP-500 has already completed Phase I clinical trials in Britain and Saudi Arabia, and the University of Oxford is now conducting a Phase Ib trial in the UK to assess vaccination of older adults, the age group most in need of this vaccine.
The VTP-500 development program was awarded PRIME designation in December 2023 by the European Medicines Agency.
This program is essential, as no vaccines targeting MERS have been licensed.
“We are thrilled to be working with the University of Oxford and CEPI on developing this important vaccine candidate,” commented Bill Enright, Barinthus Bio’s Chief Executive Officer, in a December 21, 2023 press release.
MERS is a severe respiratory infection caused by MERS-CoV, a coronavirus first identified in 2012 in Saudi Arabia. It has caused more than 2,600 human infections in at least 27 countries. MERS has a case-fatality rate of more than 35%.
The World Health Organization wrote on August 29, 2023, that it expects additional MERS-CoV cases to be reported from the Middle East and/or other countries where MERS-CoV is circulating in dromedaries.
Barinthus Bio, formerly Vaccitech plc, is a clinical-stage biopharmaceutical company developing novel T-cell immunotherapeutic candidates designed to guide the immune system to overcome chronic infectious diseases, autoimmunity, and cancer.

HOOKIPA Pharma Inc. today announced that Gilead Sciences has purchased 15 million shares of the company's common stock for approximately $21.25 million, for $1.4167 per share.
This transaction closed on December 20, 2023, indicating Gilead's total ownership in HOOKIPA's Common Stock is approximately 19.4%.
In addition, HOOKIPA has other transaction rights.
Joern Aldag, Chief Executive Officer at HOOKIPA, commented in a press release on December 21, 2023. "Together, we have made meaningful progress in finding a potential functional cure for Human Immunodeficiency Virus (HIV)."
"Most recently, we received clearance from the U.S. Food and Drug Administration of our Investigational New Drug application for HB-500 and are excited to begin our Phase 1 trial in the first half of 2024."
HB-500 is an alternating, 2-vector arenaviral therapeutic vaccine that is being evaluated as part of a potential curative regimen for HIV.
One vector is based on lymphocytic choriomeningitis virus as its arenaviral backbone; another vector is based on the Pichinde virus.
Both encode the same HIV antigens.
HOOKIPA is responsible for advancing the HIV program through the completion of a Phase 1b clinical trial. Gilead has the exclusive right to assume further development of the program thereafter.
As of December 22, 2023, there are several HIV vaccine candidates conducting research, but none have been approved. According to the U.S. National Institutes of Health, HIV vaccine candidates can not cause an HIV infection.

In Canada, most influenza surveillance indicators are increasing but remain within expected levels typical of this time of year.
From August 27, 2023, to December 9, 2023, 163 laboratory-confirmed influenza outbreaks have been reported in Canada.
From an age-group perspective, adults 65 and older have accounted for 42% of recent flu-related hospitalizations.
On December 21, 2023, Canada confirmed its recommendation for seniors to get a flu shot for the 2023-2024 season.
Canada's National Advisory Committee on Immunization (NACI) preferentially recommends adults 65 years of age and older preferentially receive an enhanced influenza vaccine, which includes adjuvanted, high-dose, and recombinant vaccines.
This included CSL Seqirus's FLUAD® Influenza vaccine (surface antigen, inactivated, adjuvanted with MF59®).
"The 2022/23 influenza season was especially challenging for Canadians and added to the already significant strain being experienced by our healthcare systems," said Bertrand Roy, Ph.D., Country Head Medical Affairs Canada at CSL Seqirus, in a press release on December 21, 2023.
The NACI's new recommendation is based on data from studies showing that all approved enhanced influenza vaccines effectively reduce the risk of influenza-related hospitalization and medical encounters.
Influenza causes an average of 12,200 hospitalizations and approximately 3,500 deaths each year in Canada, with the majority occurring in adults over 65 years of age.
Canadians over 65 accounted for 76% of the reported influenza-associated deaths during the 2022/23 flu season.

Vaxart, Inc. today announced that it has completed enrollment and dosing in the Phase 1 clinical trial evaluating Vaxart’s oral pill bivalent norovirus vaccine candidate focused on lactating mothers.
There is no U.S. Food and Drug Administration-approved vaccine against norovirus.
Outbreaks sickens approximately 21 million people in the United States annually, and 15% of children under age five contract norovirus annually.
Approximately 3 million sets of parents are forced by this virus to miss work, 2.2 days on average, to care for their children. The annual disease burden from norovirus is $10.6 billion in the U.S. alone.
“This is an important step forward as we drive toward a vaccine candidate that may make it possible for mothers to protect their children against this highly contagious – and potentially lethal -- virus. We look forward to announcing topline data from this study by the end of 2024,” commented Dr. James F. Cummings, Vaxart’s Chief Medical Officer, in a press release on December 21, 2023.
“We are very proud of our clinical team for completing enrollment of this trial within our planned timeline.”
The Phase 1, multicenter, randomized, double-blind, placebo-controlled single dose, dose-ranging study (VXA-NVV-108) is designed to evaluate the safety, tolerability, and immunogenicity of orally administered bivalent GI.1/GII.4 norovirus vaccine in healthy lactating females of at least 18 years of age.
As of late December 2023, several norovirus vaccine candidates are conducting clinical research.

Children located in a few African countries now have access to a second malaria vaccine, the R21/Matrix-M™ malaria vaccine.
As of December 21, 2023, the World Health Organization (WHO) has added this malaria vaccine to its list of prequalified vaccines, which increases access to vaccines procured by UNICEF and funding support for deployment by Gavi, the Vaccine Alliance.
In October 2023, WHO recommended that Oxford University's developed and Serum Institute of India manufactured R21/Matrix-M to prevent malaria in children following the advice of the WHO Strategic Advisory Group of Experts on Immunization and the Malaria Policy Advisory Group.
R21/Matrix-M integrates Novavax Inc.'s Matrix-M djuvant.
The initial malaria vaccine, Mosquirix™ RTS, S/AS01, obtained WHO prequalification status in July 2022.
"Today marks a huge stride in global health as we welcome the prequalification of R21/Matrix-M, the second malaria vaccine recommended for children in malaria-endemic areas, said Dr. Kate O'Brien, Director of WHO's Department of Immunization, Vaccines and Biologicals, in a press release on December 21, 2023.
"This is another step toward ensuring a healthier, more resilient future for those who have lived for too long in fear of what malaria could do to their children. Together with our partners, we are united in pursuing a malaria-free future, where every life is shielded from the threat of this disease."
Malaria is a mosquito-borne disease causing a high burden on children in the African Region, where nearly half a million children die from the disease each year. Globally, in 2022, there were an estimated 249 million malaria cases and 608,000 malaria deaths across 85 countries.
In the United States, about 2,000 malaria cases are detected in international travelers annually. However, local malaria cases in the U.S. (Arkansas, Florida, Maryland, Texas) were detected in 2023.
These WHO-prequalified malaria vaccines are not available in the U.S.
With approved Ebola vaccines and monoclonal antibody therapies available in Africa, a safe and effective treatment option is now the goal for researchers.
RedHill Biopharma Ltd. today announced that its two novel, oral host-directed investigational drugs, opaganib and RHB-107 (upamostat), demonstrated robust synergistic effect when combined individually with Veklury®, significantly improving viral inhibition while maintaining cell viability in a new U.S. Army-funded and conducted Ebola virus disease in vitro study.
Jeffrey Kugelman, Ph.D., Major(P), U.S. Army MSC, Branch Chief Synthetic Biology & Surveillance, Molecular Biology Division, U.S. Army Medical Research Institute of Infectious Diseases, who led the bioinformatics analysis of the study, commented in a press release on December 20, 2023, "The results suggest that opaganib and upamostat may have potential or use in combination with direct antiviral agents, such as Veklury, to improve treatment outcome, increasing efficacy while maintaining safety."
Opaganib (ABC294640) is a proprietary investigational host-directed and potentially broad-acting drug and is a first-in-class, orally administered sphingosine kinase-2 selective inhibitor with anticancer, anti-inflammatory, and antiviral activity, targeting multiple potential diseases.
"Opaganib is believed to be the first host-directed molecule to show activity in Ebola virus disease, and these results add to a recent U.S. Army Ebola virus study in which opaganib delivered a statistically significant increase in mice survival time in vivo," added Reza Fathi, Ph.D., RedHill's SVP R&D.
RHB-107 (upamostat) is a proprietary, first-in-class, once-daily orally administered investigational antiviral that targets human serine proteases in preparing the spike protein for viral entry into target cells. Because it is host-cell targeted, RHB-107 is expected to also be effective against emerging viral variants with mutations in the spike protein. In addition, RHB-107 inhibits several proteases targeting cancer and inflammatory gastrointestinal disease.
There are two types of Ebola causing outbreaks in Africa.
The initial Zaire Ebolavirus disease outbreak was confirmed in 1976 in South Sudan and the Democratic Republic of Congo.
African countries have endured Zaire Ebolavirus outbreaks between 2014, 2016, 2018, and 2022. Over 29,000 people were infected, and more than 11,000 died.
Separately, there have been five Sudan Ebolavirus outbreaks.
The World Health Organization issued new guidelines (August 2023) on Ebola infection prevention and control.

Merck today announced the U.S. Food and Drug Administration (FDA) has accepted for priority review a new Biologics License Application (BLA) for V116, a 21-valent pneumococcal conjugate vaccine candidate specifically designed to help prevent invasive pneumococcal disease and pneumococcal pneumonia in adults.
The FDA grants priority review to medicines and vaccines that, if approved, would significantly improve the safety or effectiveness of the treatment or prevention of a serious condition. The FDA has set a Prescription Drug User Fee Act of June 17, 2024.
The serotypes covered by V116 are responsible for approximately 83% of invasive pneumococcal disease in individuals 65 and older.
Dr. Eliav Barr, senior vice president, head of global clinical development, and chief medical officer, Merck Research Laboratories, commented in a press release on December 19, 2023, "If approved, V116 would be the first pneumococcal conjugate vaccine specifically designed to address the serotypes that cause most adult invasive pneumococcal disease."
"We look forward to discussing the data that support our filing with the FDA and are urgently working to bring this potential new preventative measure to adult patients."
Merck says V116 includes eight unique serotypes not covered by currently licensed pneumococcal vaccines, responsible for approximately 30% of invasive pneumococcal disease in individuals 65 and older.