Search API
The Novo Nordisk Foundation today announced it is committing up to $260 million to establish a state-of-the-art research and vaccine development initiative.
The aim is to create new or improved vaccines for some of the deadliest respiratory diseases, including tuberculosis, influenza, and Group A Streptococcus.
This is the first vaccine initiative globally to focus solely on understanding how to generate immunity in the airway. This is a potentially revolutionary means to block infection and prevent airborne diseases from spreading between humans.
The research arm – the Novo Nordisk Foundation Center for Vaccines and Immunity – is funded via an eight-year grant and anchored in the Department of Immunology and Microbiology at the University of Copenhagen, which has gained global recognition for its expertise in infectious disease, immunology, and technological innovation.
“Basic research carries great importance when it comes to the health and well-being of the world’s population – both present and future,” says Dean Bente M. Stallknecht from the Faculty of Health and Medical Sciences, University of Copenhagen, in a press release on December 18, 2023.
“Vaccines and knowledge of immunology is a key part of that. Boosting excellent basic research within this field of research will pave the way for discoveries and hold the potential to make a huge difference to so many people globally.”
A key partner in the initiative will be Denmark’s Statens Serum Institut.

The World Health Organization (WHO) today announced that COVAX will close at the end of 2023.
The WHO stated on December 19, 2023, COVAX, a multilateral mechanism for equitable global access to COVID-19 vaccines launched in 2020, will close on December 31, 2023, having delivered nearly 2 billion vaccines to 146 economies.
COVAX's end-to-end efforts helped lower-income economies achieve a COVID-19 two-dose coverage of 57%, compared to the global average of 67%.
In 2024 and 2025, low- and lower-middle-income economies will continue to receive COVID-19 vaccines and delivery support from Gavi, the Vaccine Alliance.
Most of COVAX's advance purchase supply agreements will have been completed or terminated by the end of 2023, except one, where a modest volume of supply will continue into the first half of 2024. So far, 58 lower-income economies have requested 83 million doses in 2024.
"COVID-19 has been the greatest health challenge of our time, and it was met with innovation and partnership on an equally unprecedented scale," said José Manuel Barroso, Chair of the Board of Gavi, the Vaccine Alliance, in a WHO press release.
"COVAX's impact has been historic, as are the insights it has generated on how, concretely, the world can do better next time."
"As we transition COVID-19 into Gavi's routine programming, we do so with deep gratitude for the passion, dedication, and sacrifice of so many around the globe who fought tirelessly for three years to try and create a more equitable world – and with an unwavering commitment to improving by transforming learnings into tangible action."
COVAX was a historic effort co-led by Gavi, the Coalition for Epidemic Preparedness Innovations, and the WHO. As of the end of 2023, the WHO has Listed twelve different COVID-19 vaccines.

GeoVax Labs, Inc. today announced that it has amended a previously executed Patent and Biological Materials License Agreement with the U.S. National Institute of Allergy and Infectious Diseases (NIAID) in support of GeoVax's development of a vaccine against the SARS-CoV-2 betacoronavirus that causes COVID-19.
The NIAID amendment expands GeoVax's commercial license to include the mpox and smallpox orthopoxviruses as additional indications.
The License Agreement, as amended, enables the creation of preventive Modified Vaccinia Ankara Virus-Virus Like Particle (MVA-VLP) vaccines that prime and/or boost the immune system against COVID-19, as well as mpox and/or smallpox.
David Dodd, GeoVax President and CEO, commented in a press release on December 19, 2023, "...The addition of the Mpox and smallpox indications to our NIAID License Agreement complements GeoVax's license agreement with City of Hope National Medical Center for GEO-CM04S1."
Mr. Dodd continued, "We anticipate that adding the Mpox/Smallpox indication to an MVA-vectored COVID-19 vaccine is a viable regulatory pathway and may be an important product differentiator from other competitors."
"For those regions/populations where Mpox and/or smallpox may be of a concern, we believe our COVID-19 vaccine will be a better choice."
Modified Vaccinia Ankara (MVA) is the vaccine currently used and stockpiled in the U.S. Strategic National Stockpile for immunization against the Mpox (JYNNEOS) and smallpox (ACAM2000) viruses.
GeoVax Labs, Inc. is a clinical-stage biotechnology company. The unedited press release is available at this link.

Novavax Inc. announced today that its updated protein-based COVID-19 vaccine is now available in France to prevent COVID-19 in individuals aged 12 and older.
As of December 19, 2023, the updated XBB version of its Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) (NVX-CoV2601), is intended for adults and adolescents aged 12 and over, regardless of their vaccination history, but is not recommended for pregnant women pending further data, according to the National recommendation of France's General Directorate of Health.
France's Ministry of Health provides the new protein-based vaccine in hospitals (December 7, 2023) and retail pharmacies (December 14, 2023).
As outlined in French vaccination recommendations, 'a diverse vaccine portfolio with both mRNA and non-mRNA options is critical to helping to protect communities across France against COVID-19 this vaccination season and in future.'
In the United States, the Food and Drug Administration amended the EUA on October 3, 2023, of the Novavax COVID-19 Vaccine, Adjuvanted, for use in individuals 12 and older, to include the 2023-2024 formula.
As of December 19, 2023, Novavax's protein-based vaccine is the only non-mRNA COVID-19 vaccine available in the U.S.

Invivyd, Inc. today announced positive initial results from the ongoing CANOPY Phase 3 pivotal clinical trial of VYD222, a broadly neutralizing, half-life extended monoclonal antibody (mAb) candidate for the prevention of symptomatic COVID-19.
Results showed that the safety and tolerability profile of VYD222 remains favorable, with no study drug-related serious adverse events reported to date.
The company also reported on December 14, 2023, that in vitro pseudovirus testing shows VYD222 has potency against various SARS-CoV-2 coronavirus variants currently circulating, such as HV.1, BA.2.86, XBB.1.5.10/EG.5, and HK.3.
Importantly, VYD222 continues to show neutralizing activity against variants with the F456L mutation found in most variants in the U.S.
Currently, no mAb is authorized or approved in the U.S. to prevent symptomatic COVID-19.
"We are pleased to share positive initial topline results from CANOPY, which bolster our belief that VYD222 holds the potential to provide vulnerable people, particularly the immunocompromised (IC), with meaningful protection from COVID-19," said Dave Hering, Chief Executive Officer of Invivyd, in a press release.
"VYD222 produced high serum virus neutralizing antibody (sVNA) titer levels against XBB.1.5 in the IC cohort, essentially replicating the titer levels observed in our Phase 1 clinical trial of VYD222 in healthy volunteers."
"We are also encouraged by the potential early signal of strong clinical protection from symptomatic COVID-19 in the CANOPY clinical trial to date, which would be expected given the high VYD222 sVNA titer levels and dose selected."
"We look forward to continued engagement with the FDA on these promising results, and we intend to submit a request for Emergency Use Authorization as soon as practicable."
Globally, there are millions of immunocompromised people, with more than 9 million in the U.S. alone who may not adequately respond to COVID-19 vaccination.

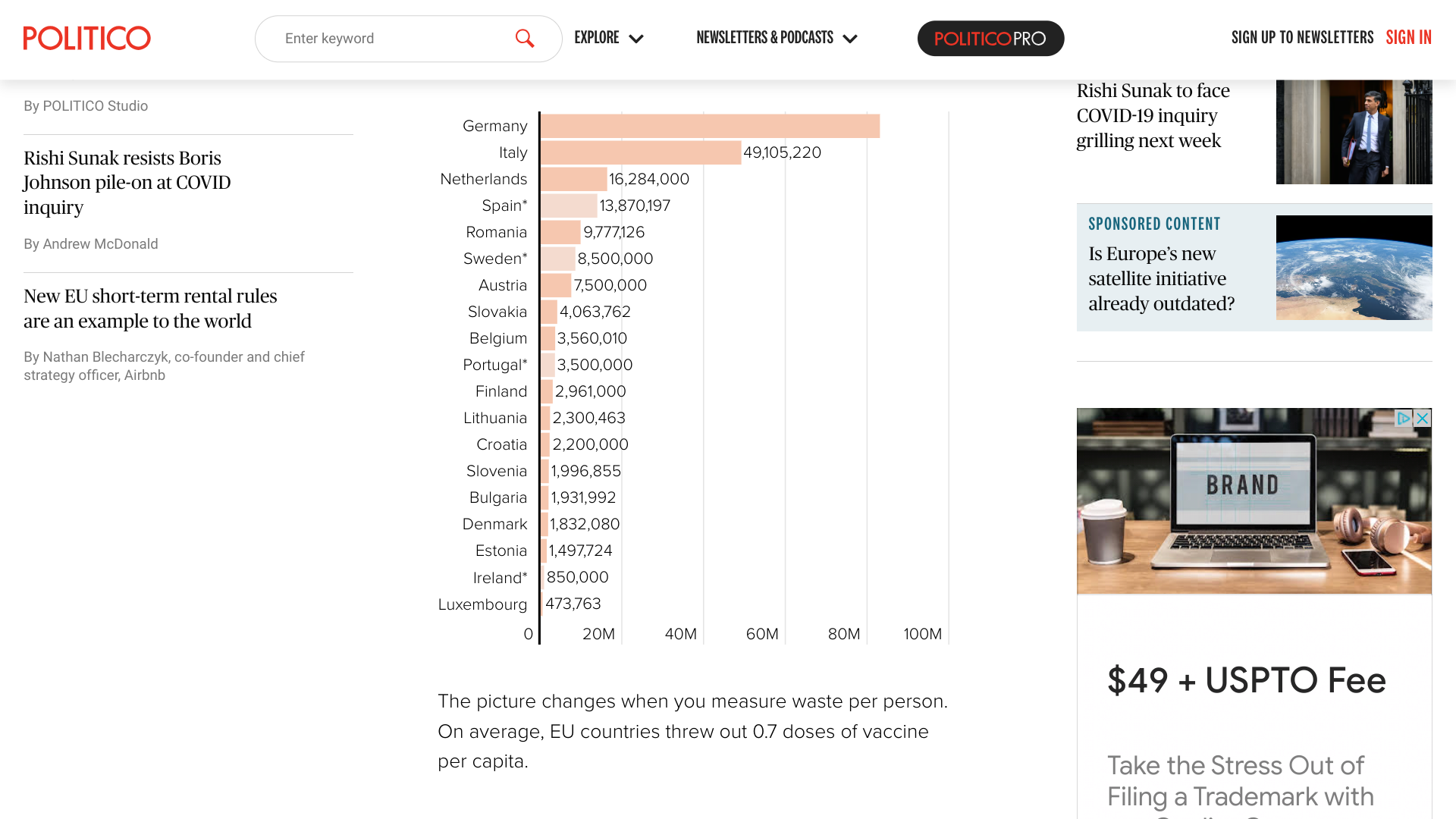
POLITICO reported today at least 215 million COVID-19 vaccines purchased by European Union (EU) countries have been recently discarded at an estimated cost of $4.3 billion.
Since COVID-19 vaccines became available in late 2020, EU countries have collectively taken delivery of 1.5 billion doses.
POLITICO's December 18, 2023 announcement is based on data from 19 EU countries. Germany, for example, provided its vaccine waste figures to POLITICO in June 2023.
As 2024 approaches, purchases for updated COVID-19 vaccines continue.
On December 13, 2023, the World Health Organization (WHO) announced that given the current SARS-CoV-2 betacoronavirus evolution and the breadth in immune responses demonstrated by monovalent XBB.1.5 vaccines, the Technical Advisory Group on COVID-19 Vaccine Composition advises retaining the current COVID-19 vaccine antigen composition (monovalent XBB.1.5) into 2024.
There are 12 COVID-19 vaccines currently Listed by the WHO.
In the U.S., only three vaccines are available.
As of December 2, 2023, only 7.7% of children were up to date with the 2023-24 COVID-19 vaccine, as reported by the U.S. CDC.
And 17.2% of adults reported receiving an updated 2023-24 COVID-19 vaccine since September 14, 2023.
In the U.S., 2023-24 COVID-19 vaccine coverage data using interactive maps, trend lines, bar charts, and tables are available at this CDC link.
The U.S. National Center for Health Statistics (NCHS) Mortality Surveillance mortality surveillance data indicates that 0.2% of deaths during the week ending December 9, 2023 (Week 49) were due to influenza.
This NCHS percentage remained stable compared to Week 48.
Unfortunately, the U.S. CDC reported two additional influenza-associated pediatric deaths last week.
As of December 14, 2023, a total of 14 influenza-associated pediatric deaths, vaccination status not reported, have occurred during the 2023-2024 flu season.
The CDC reported seasonal influenza activity remains elevated in most parts of the country and recommends that most people over six months get an annual flu shot.
Furthermore, based on when you get a flu shot, your health condition, and where you live, a second vaccination may be appropriate based on a conversation with a doctor, nurse, or pharmacist.
During this flu season, over 152 million nasal, egg-based, and cell-based influenza vaccines were distributed in the U.S. These vaccines remain available at most pharmacies.

According to several health agencies, the global risk assessment related to dengue virus outbreaks increased in late December 2023.
The European Centre for Disease Prevention and Control reported that as of November 2023, over 4.5 million cases and over 4,000 dengue-related deaths have been reported from 80 countries/territories globally.
For example, the U.S. CDC reissued Level 1 - Practice Usual Precautions, Travel Health Notices on December 14, 2023, for countries located in Africa, Asia, the Americas, the Middle East, and the Pacific Islands.
In the Americas, the Pan American Health Organization (PAHO) confirmed that 2023 is the year with the highest historical record of dengue cases, registering more than 4.1 million new infections, exceeding 2019, when about 3.1 million cases were reported.
Several factors are associated with the increasing risk of dengue epidemics, such as the changing range in elevation of Aedes aegypti mosquitoes, especially in previously dengue native areas, stated the PAHO on December 12, 2023.
Because mosquito bites spread dengue, all travelers to these areas, including southeast Florida and Puerto Rico, are at risk.
Since dengue can become severe within a few hours, usually requiring hospitalization, prevention options are essential.
In addition to avoiding mosquito bites, dengue is a vaccine-preventable disease.
As of late 2023, two approved dengue vaccines and several vaccine candidates are in development. These vaccines have limited availability, based on which country you are located in.


