Search API
Jiangsu Recbio Technology Co., Ltd. today announced that the novel adjuvanted recombinant shingles vaccine candidate REC610 recently achieved positive results in the interim analysis of the first-in-human clinical trial in the Philippines.
Published on December 29, 2023, the Interim Analysis results showed that REC610 demonstrated an overall favorable safety and tolerability profile in healthy participants aged 40 and above after two vaccination doses.
REC610 induced strong gE-specific humoral and cellular immune responses, which were evident after the first vaccination and peaked 30 days after the second vaccination.
The humoral and cellular immune responses were comparable between REC610 and the Shingrix® vaccine comparison group, and the immune response level in the REC610 group was numerically higher than that in the Shingrix group.
REC610 is intended to prevent shingles in adults aged 40 and above. It is equipped with a novel adjuvant BFA01 independently developed by the Company, which can promote the production of high levels of VZV glycoprotein E (gE)-specific CD4+ T cells and antibodies.
REC610 received a drug clinical trial approval notice issued by the National Medical Products Administration in October 2023. It is approved as a preventive 3.3 biological product in its Phase I and III clinical trials in China.
The Company will soon adopt a randomized, double-blind, parallel controlled phase I clinical trial in 180 healthy adult subjects aged 40 and above in Mainland China to evaluate the safety, tolerability, and immunogenicity of REC610.
According to statistics, China's population aged 40 and above is approximately 700 million, and about 6 million new shingles cases occur each year in China.
Furthermore, the incidence of shingles has gradually become younger in recent years.
Only Shingrix is on the market in China, and there is a strong demand for import substitution, according to Jiangsu Recbio Technology's press release.
Beginning in January 2024, Chongqing Zhifei Biological Products will have exclusive rights to import and distribute Shingrix in China.

The World Health Organization (WHO) recently published its External Situation Report #31 regarding the ongoing, multi-country outbreak of mpox.
Based on the data reported on December 22, 2023, the mpox outbreak continues in most WHO regions, while more extensive transmission has been observed in the European Region and the Region of the Americas.
A total of 906 additional mpox cases were reported in November 2023, representing a 26% increase compared to October.
The United States of America (299 vs. 135) reported the most significant increase in the Americas. The states of California, New York, and Texas have reported the most mpox cases.
Around half (52%) of cases with available information in this outbreak are reported to be in persons living with HIV.
The U.S. CDC's Advisory Committee on Immunization Practices voted on October 25, 2023, to recommend the routine use of the JYNNEOS® vaccine for people at risk of mpox infection.
However, only 23% of the at-risk population has been fully vaccinated nationally as of December 2023.
On December 7, 2023, the CDC published an advisory stating the JYNNEOS is expected to be effective for both Clade I and Clade II MPXV infections. However, real-world data is lacking, reported the European CDC.
According to the CDC, approximately 1.2 million JYNNEOS vaccine doses have been administered in the U.S. since the mpox outbreak began in May 2022.
Despite a global reduction in Zika cases since a peak in 2017, the Pan American Health Organization (PAHO) recently confirmed the circulation of this mosquito-borne virus in 89 countries worldwide.
However, Zika outbreaks escalated in certain countries in the Region of the Americas in 2023.
The PAHO's data dashboard indicates there have been 35,538 confirmed Zika cases this year.
Ten countries, led by Brazil, have accounted for about 89% of Zika cases recorded over the past decade.
This year, Brazil has reported over 33,000 Zika cases.
In the United States, the Centers for Disease Control and Prevention (CDC) says mosquitoes continue to spread Zika throughout Puerto Rico.
About 25% of infected people develop Zika symptoms, and the illness is usually mild, lasting between two and seven days.
Moreover, Congenital Zika-associated syndrome is a set of anomalies, such as microcephaly, seen in infants born to mothers with a history of gestational Zika fever, says the CDC.
The PAHO and other health agencies have stated that vaccination is the best option to prevent further infections.
But, as 2024 approaches, no Zika vaccine has been approved by any health authority.
Clinical trials involving DNA, modified vaccinia Ankara vector platform, and purified inactivated virus vaccine candidates have shown they can induce neutralizing antibodies.
Since 2016, about $350 million of funding has been allocated for Zika vaccine candidates.

According to local media, Brazil's Ministry of Health announced it would integrate the Qdenga® tetravalent vaccine into its Unified Health System (SUS).
The two-dose, 0.5 mL each, three months apart, Qdenga vaccinations are scheduled to launch in February 2024.
About 5 million vaccinations in 2024 will be focused on specific audiences and priority regions without pre-administration testing.
"The Ministry of Health assessed the cost-benefit relationship and the issue of access, as in a country like Brazil, it is necessary to have an adequate quantity of vaccines for the size of our population. Following the favorable opinion of Conitec, we will be the first country to provide public access to this vaccine as an immunizer under SUS," said Health Minister Nísia Trindade, reported Folha de Paulo on December 22, 2023.
The World Health Organization recently confirmed dengue is endemic in about 125 countries.
During 2023, the most significant number of dengue outbreaks were reported in Brazil, with over 2.5 million patients.
Qdenga was initially authorized in Indonesia, followed by Argentina, Denmark, Germany, Portugal, and Thailand. This dengue vaccine is not approved in the U.S.
Note: This article was updated for clinical accuracy on January 10, 2024.

Based on last week's airport screening activity, the U.S. Transportation Security Administration (TSA) is expecting a busy end-of-year holiday season.
As of December 25, 2023, the number of air travelers screened exceeded the volume last seen in 20219.
And to keep the wait times down, about 99% of TSA PreCheck® passengers waited less than 10 minutes passing through security lines in early December 2023.
Travelers can also help keep screening wait times down by remembering to bring an acceptable ID, including a digital wallet.
In collaboration with the State of Arizona and Samsung, the TSA is now accepting, for limited testing and evaluation purposes, Arizona-issued mobile driver's licenses and identification cards in Samsung Wallet on Samsung mobile devices at select TSA airport security checkpoints.
Passengers can use this new feature at checkpoints for identity verification at 27 participating airports.
TSA introduced the concept of the mobile driver's license in April 2021. Since then, Google and Apple digital wallets have also been accepted at various airports in the U.S.
This is one of TSA's many steps to transform airport security experiences.
From a health perspective, the U.S. CDC recently issued Travel Health Notices regarding dengue virus outbreaks in numberious countries.
The CDC says that getting vaccinated against infectious diseases is one of the most effective ways to protect your health while traveling abroad.
Travel vaccines can prevent dengue and other infectious diseases and are generally available at travel clinics and pharmacies in the U.S.

The U.S. Centers for Disease Control and Prevention (CDC) reported on December 22, 2023, the updated percentage of people who have received COVID-19, influenza, and/or Respiratory syncytial virus (RSV) vaccines in 2023.
Influenza, the SARS-CoV-2 coronavirus, and RSV have been circulating during the fall through early spring for the past few years, causing respiratory illness, says the CDC.
Alaska recently reported particularly low vaccination rates for the three illnesses that typically send hundreds of Alaskans to the hospital yearly.
Dr. Joe McLaughlin, Alaska’s state epidemiologist, informed Anchorage Daily News fewer than 20% of Alaskans had gotten a flu shot, and just 13% of eligible Alaskans were up-to-date on their COVID-19 vaccinations.
“I’m always concerned about vaccination rates,” McLaughlin said, noting that Alaska often has one of the lower vaccination rates in the country for flu and other illnesses.
“This year, our coverage rates are below even what they have been in recent years.”
Regarding the new COVID-19 vaccines, the CDC reported that 7.6% (95% confidence interval: 6.7-8.4) of children and 18.5% (17.8-19.2) of adults have been vaccinated.
The percent of the population reporting receipt of a flu vaccine is about 43%
The percentage of adults 60+ reported receiving an RSV vaccine is 17% (15.9-18.1).
These respiratory vaccines remain available at most pharmacies in the U.S.
In late 2023, the CDC confirmed partnering with community-based organizations, healthcare providers, and other trusted messengers to build vaccine confidence and awareness.
Since egg, cell, and nasal-based flu shots are available, consumers have ample choice to select which vaccine is best for them.

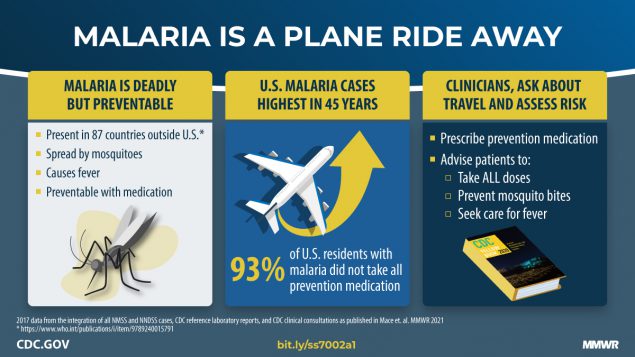
UNICEF recently confirmed that more than 550,000 doses of a World Health Organization (WHO)-recommended malaria vaccines arrived in the Republic of Sierra Leone.
Malaria burden is significant in Sierra Leone as it accounts for over two million hospital visits per year, of which children under five years of age account for one million of these cases.
This vaccine-preventable disease accounts for about 25% of child deaths in this West African country.
These vaccinations are essential as exposure to malaria parasites does not confer lifelong protection, unlike other mosquito-transmitted diseases.
Announced on December 15, 2023, this shipment, worth $ 5.5 million, follows the shipment of over 330,000 doses to Cameroon. These shipments signal the scale-up of vaccination against malaria across the highest-risk areas on the African continent.
As of December 24, the WHO and the European Medicines Agency recommended the Mosquirix™ (RTS,S/AS01) and R21 / Matrix-M™ malaria vaccines. These vaccines have been added to the WHO's list of prequalified vaccines.
These malaria vaccines are not yet available in the Region of the Americas, which includes the United States.
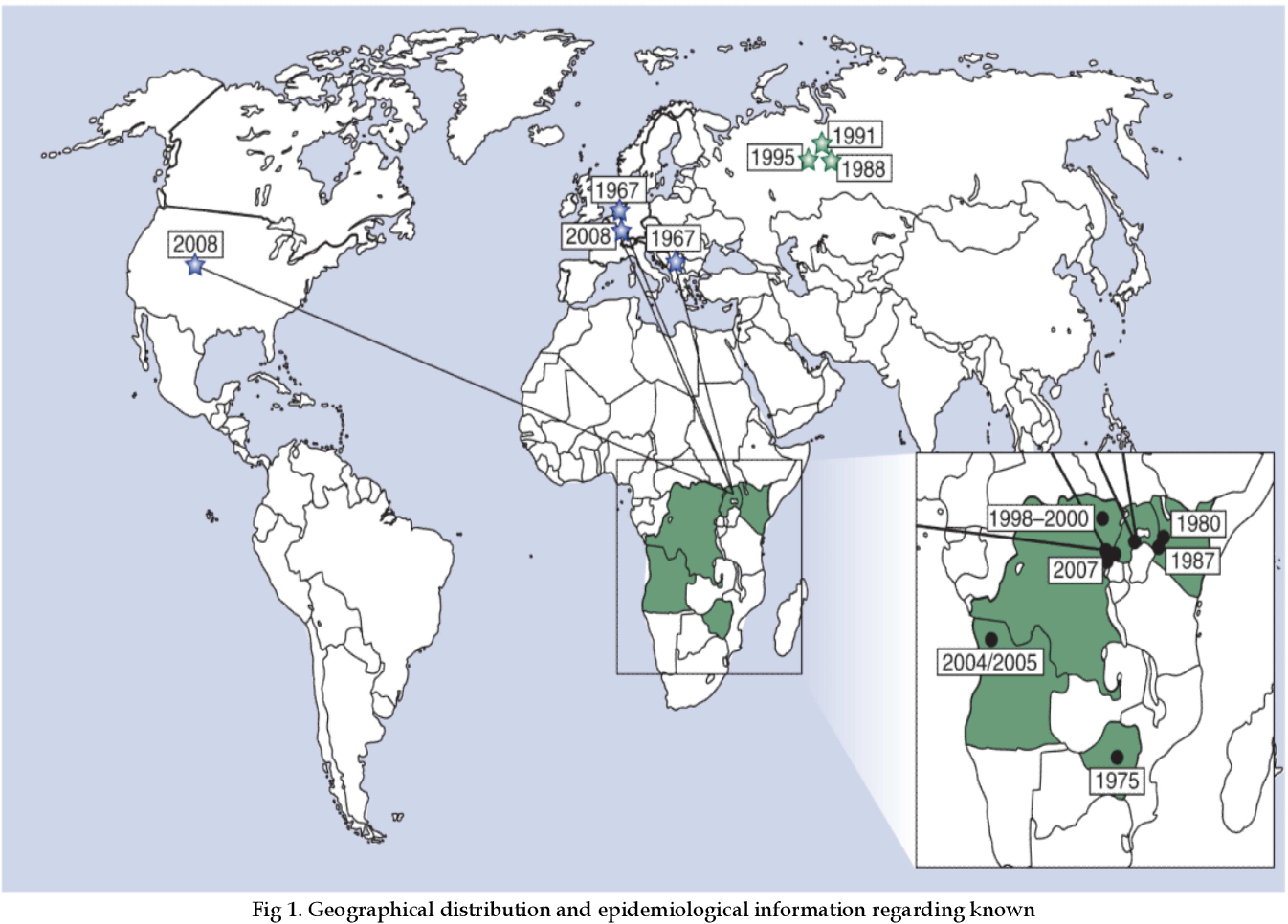
First identified in Germany in 1967, Marburg virus (MARV) outbreaks have been reported more than a dozen times over the past 56 years.
A Marburg virus (MARV) vaccine tested at Texas Biomedical Research Institute is progressing in clinical trials, moving a step closer to becoming the world’s first vaccine against the deadly virus.
The Sabin Vaccine Institute recently announced it launched Phase 2 clinical trials of its single-dose vaccine candidate ChAd3-MARV.
Early tests demonstrating the vaccine’s efficacy, safety, and optimal dosage were completed at Texas Biomed.
This announcement is essential since there are no approved Marburg vaccines or treatments.
“We have been partnering with Sabin since 2019 and are very excited to see their Marburg vaccine candidate move into Phase 2 clinical trials,” says Ricardo Carrion, Jr., Ph.D., the Director of Maximum Containment Contract Research at Texas Biomed, in a press release on December 7, 2023.
“An effective vaccine is critical to protect people from this deadly virus, especially as we see the frequency of outbreaks increasing in more places.”
The Phase 2 clinical trial will build on promising results from preclinical studies and a smaller Phase 1 clinical trial.
Texas Biomed continues to partner with Sabin to gather more detailed information that can only be gained through tightly controlled animal studies, including how soon protection is induced after vaccination.
Marburg virus is a part of the same filovirus family as Ebola virus and causes severe hemorrhagic fever. It is incredibly deadly, with up to a 90% fatality rate.
Recent MARV outbreaks that occurred in Equatorial Guinea killed 12 out of 17 confirmed cases, with another 23 probable deaths, according to the World Health Organization.
Tanzania also saw its first-ever Marburg outbreak, which killed six out of eight confirmed cases.
Texas Biomed has conducted similar work on Sabin’s closely related Sudan ebolavirus vaccine as part of a World Health Organization-coordinated outbreak response.
As of December 24, 2023, several Ebola vaccines, therapies, and vaccine candidates are conducting research studies. Ebola vaccines are not generally available in the U.S.
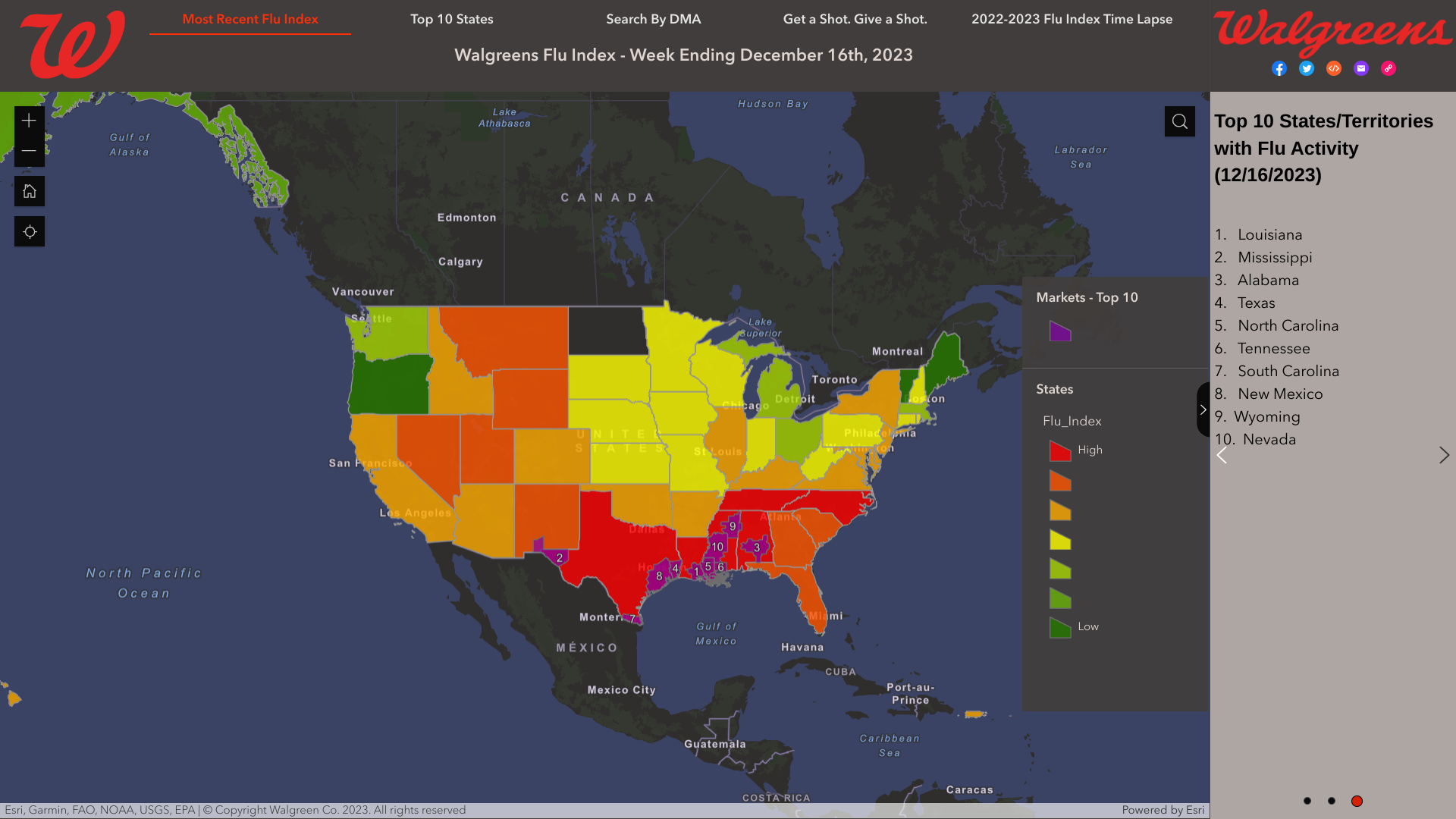
Most scientists say that having two independent data points empowers better decisions. This philosophy can be applied when determining the local health risk during the 2023-2024 flu season.
As of late December 2023, the U.S. Centers for Disease Control and Prevention (CDC) and Walgreens published updated maps and data identifying where influenza is being detected.
The CDC's FluView Updates for Week #50, ending December 16, 2023, reported seasonal influenza activity was elevated and continues to increase in most parts of the country.
And outpatient respiratory illness is above baseline for the 7th consecutive week and is above baseline in all 10 HHS Regions.
Previously, the Walgreens Flu Index® reported state and market-specific information regarding flu activity, which is compiled using retail prescription data for antiviral medications used to treat influenza across Walgreens locations nationwide.
As of December 16, 2023, Walgreens identified the top ten states:
- Louisiana
- Mississippi
- Alabama
- Texas
- North Carolina
- Tennessee
- South Carolina
- New Mexico
- Wyoming
- Nevada
The good news from the CDC is that as of December 2023, over 153 million influenza vaccines were distributed this season, available today at most pharmacies in the U.S.