Search API
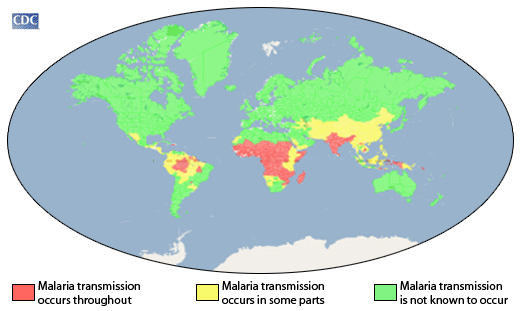
Children located in a few African countries now have access to a second malaria vaccine, the R21/Matrix-M™ malaria vaccine.
As of December 21, 2023, the World Health Organization (WHO) has added this malaria vaccine to its list of prequalified vaccines, which increases access to vaccines procured by UNICEF and funding support for deployment by Gavi, the Vaccine Alliance.
In October 2023, WHO recommended that Oxford University's developed and Serum Institute of India manufactured R21/Matrix-M to prevent malaria in children following the advice of the WHO Strategic Advisory Group of Experts on Immunization and the Malaria Policy Advisory Group.
R21/Matrix-M integrates Novavax Inc.'s Matrix-M djuvant.
The initial malaria vaccine, Mosquirix™ RTS, S/AS01, obtained WHO prequalification status in July 2022.
"Today marks a huge stride in global health as we welcome the prequalification of R21/Matrix-M, the second malaria vaccine recommended for children in malaria-endemic areas, said Dr. Kate O'Brien, Director of WHO's Department of Immunization, Vaccines and Biologicals, in a press release on December 21, 2023.
"This is another step toward ensuring a healthier, more resilient future for those who have lived for too long in fear of what malaria could do to their children. Together with our partners, we are united in pursuing a malaria-free future, where every life is shielded from the threat of this disease."
Malaria is a mosquito-borne disease causing a high burden on children in the African Region, where nearly half a million children die from the disease each year. Globally, in 2022, there were an estimated 249 million malaria cases and 608,000 malaria deaths across 85 countries.
In the United States, about 2,000 malaria cases are detected in international travelers annually. However, local malaria cases in the U.S. (Arkansas, Florida, Maryland, Texas) were detected in 2023.
These WHO-prequalified malaria vaccines are not available in the U.S.
With approved Ebola vaccines and monoclonal antibody therapies available in Africa, a safe and effective treatment option is now the goal for researchers.
RedHill Biopharma Ltd. today announced that its two novel, oral host-directed investigational drugs, opaganib and RHB-107 (upamostat), demonstrated robust synergistic effect when combined individually with Veklury®, significantly improving viral inhibition while maintaining cell viability in a new U.S. Army-funded and conducted Ebola virus disease in vitro study.
Jeffrey Kugelman, Ph.D., Major(P), U.S. Army MSC, Branch Chief Synthetic Biology & Surveillance, Molecular Biology Division, U.S. Army Medical Research Institute of Infectious Diseases, who led the bioinformatics analysis of the study, commented in a press release on December 20, 2023, "The results suggest that opaganib and upamostat may have potential or use in combination with direct antiviral agents, such as Veklury, to improve treatment outcome, increasing efficacy while maintaining safety."
Opaganib (ABC294640) is a proprietary investigational host-directed and potentially broad-acting drug and is a first-in-class, orally administered sphingosine kinase-2 selective inhibitor with anticancer, anti-inflammatory, and antiviral activity, targeting multiple potential diseases.
"Opaganib is believed to be the first host-directed molecule to show activity in Ebola virus disease, and these results add to a recent U.S. Army Ebola virus study in which opaganib delivered a statistically significant increase in mice survival time in vivo," added Reza Fathi, Ph.D., RedHill's SVP R&D.
RHB-107 (upamostat) is a proprietary, first-in-class, once-daily orally administered investigational antiviral that targets human serine proteases in preparing the spike protein for viral entry into target cells. Because it is host-cell targeted, RHB-107 is expected to also be effective against emerging viral variants with mutations in the spike protein. In addition, RHB-107 inhibits several proteases targeting cancer and inflammatory gastrointestinal disease.
There are two types of Ebola causing outbreaks in Africa.
The initial Zaire Ebolavirus disease outbreak was confirmed in 1976 in South Sudan and the Democratic Republic of Congo.
African countries have endured Zaire Ebolavirus outbreaks between 2014, 2016, 2018, and 2022. Over 29,000 people were infected, and more than 11,000 died.
Separately, there have been five Sudan Ebolavirus outbreaks.
The World Health Organization issued new guidelines (August 2023) on Ebola infection prevention and control.

Merck today announced the U.S. Food and Drug Administration (FDA) has accepted for priority review a new Biologics License Application (BLA) for V116, a 21-valent pneumococcal conjugate vaccine candidate specifically designed to help prevent invasive pneumococcal disease and pneumococcal pneumonia in adults.
The FDA grants priority review to medicines and vaccines that, if approved, would significantly improve the safety or effectiveness of the treatment or prevention of a serious condition. The FDA has set a Prescription Drug User Fee Act of June 17, 2024.
The serotypes covered by V116 are responsible for approximately 83% of invasive pneumococcal disease in individuals 65 and older.
Dr. Eliav Barr, senior vice president, head of global clinical development, and chief medical officer, Merck Research Laboratories, commented in a press release on December 19, 2023, "If approved, V116 would be the first pneumococcal conjugate vaccine specifically designed to address the serotypes that cause most adult invasive pneumococcal disease."
"We look forward to discussing the data that support our filing with the FDA and are urgently working to bring this potential new preventative measure to adult patients."
Merck says V116 includes eight unique serotypes not covered by currently licensed pneumococcal vaccines, responsible for approximately 30% of invasive pneumococcal disease in individuals 65 and older.

Uvax Bio, LLC today announced acknowledgment from the Australian Therapeutic Goods Administration and approval from the Human Research Ethics Committee (HREC) to conduct a Phase 1 study of Uvax Bio's HIV-1 vaccine candidates in Australia.
The two vaccines being tested are based on Uvax Bio's proprietary 1c-SApNP® technology, displaying 20 uncleaved, prefusion-optimized (UFO) HIV envelope (Env) trimers in wild-type and glycan-trimmed forms (UVAX-1197 and UVAX-1107, respectively).
Uvax Bio's HIV-1 vaccines are combined with Dynavax's CpG 1018® adjuvant and aluminum hydroxide.
Uvax Bio will work with their Australia-based clinical research partners Avance Clinical and the Nucleus Network study site to initiate this study in January 2024.
Previously, in a preclinical toxicology study, UVAX-1107 & 1197 combined with CpG 1018® and aluminum hydroxide were safe with no serious adverse events.
In a second preclinical immunogenicity study, immunization with Uvax Bio's HIV-1 vaccine candidates elicited robust neutralizing antibody responses against the vaccine-matched virus in 99% of the animals.
Furthermore, preliminary screening assays demonstrated appreciable neutralization in serum when tested against a panel of primary HIV-1 isolates.
"The body of evidence from our preclinical studies and GMP manufacturing runs was instrumental in facilitating this authorization to begin preparation for our first Phase 1 trial," commented Ji Li, Ph.D., Uvax Bio CEO, in a press release on December 19, 2023.
"Our clinical team will begin preparing to initiate this trial in January 2024."
As of December 20, 2023, the U.S. Food and Drug Administration, Japan's National Institute of Infectious Diseases, the European Medicines Agency, and the United Kingdom had not approved an HIV prevention vaccine.

Researchers recently wrote in an original research article that getting an annual flu shot is highly recommended. Still, influenza vaccines do not work as well in older adults due to the aging of their immune systems.
One approach to improving influenza vaccine efficacy is the addition of an adjuvant to the vaccine to boost an individual's immune response.
Published on December 19, 2023, this new study evaluated an adjuvanted vaccine compared to an unadjuvanted vaccine for preventing cardiorespiratory hospitalizations and hospitalization costs.
The Original Research's findings demonstrated that the adjuvanted flu vaccine, compared to the unadjuvanted vaccine, prevented more hospitalizations and significantly reduced associated hospital costs.
The study included 715,807 aIIV3 and 320,991 IIV4e recipients in the 2018–19 and 844,169 aIIV3 and 306,270 IIV4e recipients in the 2019–20 influenza seasons.
aIIV3 vaccination was significantly more effective than IIV4e in preventing cardiorespiratory disease (2018–19 rVE = 6.2%; and 2019–20 rVE = 6.0%) and respiratory disease (2018–19 rVE = 8.9%; and 2019–20 rVE = 10.1%).
During the 2018–19 flu season, cardiorespiratory hospitalization cost savings for the aIIV3 population were $392 million and $ 221 million for the 2019–20 season.
Respiratory hospitalization cost savings for the aIIV3 population were $145 million and $97 million, respectively.
As of December 20, 2023, over 153 million influenza vaccines (nasal, egg-based, cell-based) were distributed this flu season in the U.S. These vaccines are generally available at clinics and local pharmacies.

The Novo Nordisk Foundation today announced it is committing up to $260 million to establish a state-of-the-art research and vaccine development initiative.
The aim is to create new or improved vaccines for some of the deadliest respiratory diseases, including tuberculosis, influenza, and Group A Streptococcus.
This is the first vaccine initiative globally to focus solely on understanding how to generate immunity in the airway. This is a potentially revolutionary means to block infection and prevent airborne diseases from spreading between humans.
The research arm – the Novo Nordisk Foundation Center for Vaccines and Immunity – is funded via an eight-year grant and anchored in the Department of Immunology and Microbiology at the University of Copenhagen, which has gained global recognition for its expertise in infectious disease, immunology, and technological innovation.
“Basic research carries great importance when it comes to the health and well-being of the world’s population – both present and future,” says Dean Bente M. Stallknecht from the Faculty of Health and Medical Sciences, University of Copenhagen, in a press release on December 18, 2023.
“Vaccines and knowledge of immunology is a key part of that. Boosting excellent basic research within this field of research will pave the way for discoveries and hold the potential to make a huge difference to so many people globally.”
A key partner in the initiative will be Denmark’s Statens Serum Institut.

The World Health Organization (WHO) today announced that COVAX will close at the end of 2023.
The WHO stated on December 19, 2023, COVAX, a multilateral mechanism for equitable global access to COVID-19 vaccines launched in 2020, will close on December 31, 2023, having delivered nearly 2 billion vaccines to 146 economies.
COVAX's end-to-end efforts helped lower-income economies achieve a COVID-19 two-dose coverage of 57%, compared to the global average of 67%.
In 2024 and 2025, low- and lower-middle-income economies will continue to receive COVID-19 vaccines and delivery support from Gavi, the Vaccine Alliance.
Most of COVAX's advance purchase supply agreements will have been completed or terminated by the end of 2023, except one, where a modest volume of supply will continue into the first half of 2024. So far, 58 lower-income economies have requested 83 million doses in 2024.
"COVID-19 has been the greatest health challenge of our time, and it was met with innovation and partnership on an equally unprecedented scale," said José Manuel Barroso, Chair of the Board of Gavi, the Vaccine Alliance, in a WHO press release.
"COVAX's impact has been historic, as are the insights it has generated on how, concretely, the world can do better next time."
"As we transition COVID-19 into Gavi's routine programming, we do so with deep gratitude for the passion, dedication, and sacrifice of so many around the globe who fought tirelessly for three years to try and create a more equitable world – and with an unwavering commitment to improving by transforming learnings into tangible action."
COVAX was a historic effort co-led by Gavi, the Coalition for Epidemic Preparedness Innovations, and the WHO. As of the end of 2023, the WHO has Listed twelve different COVID-19 vaccines.

GeoVax Labs, Inc. today announced that it has amended a previously executed Patent and Biological Materials License Agreement with the U.S. National Institute of Allergy and Infectious Diseases (NIAID) in support of GeoVax's development of a vaccine against the SARS-CoV-2 betacoronavirus that causes COVID-19.
The NIAID amendment expands GeoVax's commercial license to include the mpox and smallpox orthopoxviruses as additional indications.
The License Agreement, as amended, enables the creation of preventive Modified Vaccinia Ankara Virus-Virus Like Particle (MVA-VLP) vaccines that prime and/or boost the immune system against COVID-19, as well as mpox and/or smallpox.
David Dodd, GeoVax President and CEO, commented in a press release on December 19, 2023, "...The addition of the Mpox and smallpox indications to our NIAID License Agreement complements GeoVax's license agreement with City of Hope National Medical Center for GEO-CM04S1."
Mr. Dodd continued, "We anticipate that adding the Mpox/Smallpox indication to an MVA-vectored COVID-19 vaccine is a viable regulatory pathway and may be an important product differentiator from other competitors."
"For those regions/populations where Mpox and/or smallpox may be of a concern, we believe our COVID-19 vaccine will be a better choice."
Modified Vaccinia Ankara (MVA) is the vaccine currently used and stockpiled in the U.S. Strategic National Stockpile for immunization against the Mpox (JYNNEOS) and smallpox (ACAM2000) viruses.
GeoVax Labs, Inc. is a clinical-stage biotechnology company. The unedited press release is available at this link.

Novavax Inc. announced today that its updated protein-based COVID-19 vaccine is now available in France to prevent COVID-19 in individuals aged 12 and older.
As of December 19, 2023, the updated XBB version of its Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) (NVX-CoV2601), is intended for adults and adolescents aged 12 and over, regardless of their vaccination history, but is not recommended for pregnant women pending further data, according to the National recommendation of France's General Directorate of Health.
France's Ministry of Health provides the new protein-based vaccine in hospitals (December 7, 2023) and retail pharmacies (December 14, 2023).
As outlined in French vaccination recommendations, 'a diverse vaccine portfolio with both mRNA and non-mRNA options is critical to helping to protect communities across France against COVID-19 this vaccination season and in future.'
In the United States, the Food and Drug Administration amended the EUA on October 3, 2023, of the Novavax COVID-19 Vaccine, Adjuvanted, for use in individuals 12 and older, to include the 2023-2024 formula.
As of December 19, 2023, Novavax's protein-based vaccine is the only non-mRNA COVID-19 vaccine available in the U.S.

