Search API
According to Paraguay's National Animal Health and Quality Service President, Dr. José Carlos Martin, on May 22, 2023, three confirmed outbreaks of avian influenza (bird flu) in the Chaco region occurred, while two others were still under investigation.
MercoPress, a news agency based in Uruguay, reported bird flu was registered in home-breeding establishments with open-air sheds and backyard poultry, in Mariscal Estigarribia, Colonia Neuland, and Filadelfia, in the department of Boquerón.
On May 18, 2023, the Pan American Health Organization announced bird flu outbreaks are mainly occurring in areas along the Pacific flyway and that outbreaks have occurred in 15 countries in Latin America and the Caribbean, which it said is unprecedented.
In North America, Canada and the United States have been battling the virus since early 2022 and have reported it in wild birds, poultry, and mammals.
Additional bird flu outbreak news regarding mammals and people is posted at Precision Vaccinations.
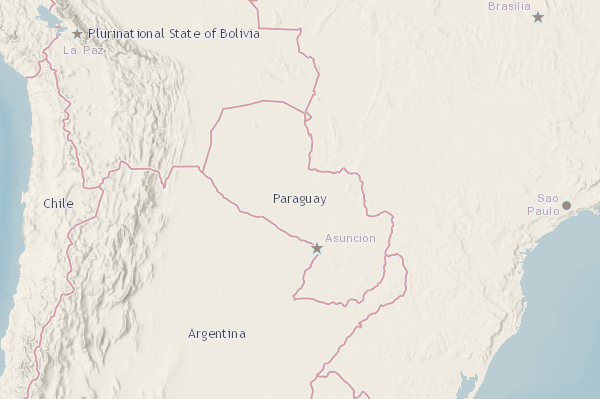
The U.S. Department of State recently reissued its Level 3 travel advisory for the Federal Democratic Republic of Ethiopia with updates to security information.
As of May 19, 2023, the U.S. says to reconsider travel to Ethiopia in cities and border areas due to sporadic civil unrest, crime, and communications disruptions.
U.S. officials have limited ability to provide services to U.S. citizens outside of Addis Ababa and any U.S. citizen detained by Ethiopian authorities.
And the government of Ethiopia has previously restricted or shut down internet, cellular data, and phone services before, during, and after civil unrest. These restrictions impede the U.S.
Please contact the Embassy’s American Citizen Services Unit at [email protected] for further assistance, and enroll in STEP to be located during emergencies.
When visiting Ethiopia, Do Not Travel To the following areas:
- Tigray Region and the border with Eritrea.
- Afar-Tigray border areas.
- Amhara Region.
- Gambella and Benishangul Gumuz Regions.
- Oromia Region.
- Southern Nations and National People Region.
- The border area with Somalia.
- Border areas with Sudan and South Sudan.
- Border areas with Kenya.
From a health perspective, the U.S. CDC suggests visitors ensure they are protected against measles and polio.
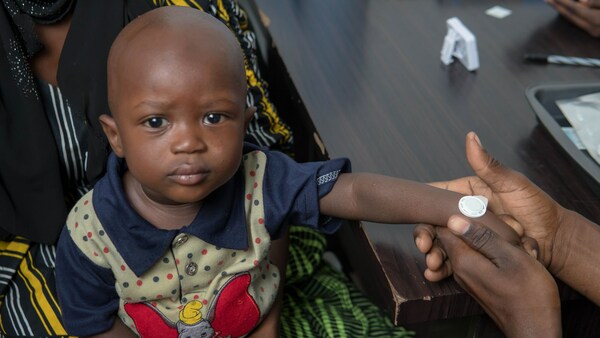
Micron Biomedical recently announced positive Phase 1/2 data from the first-ever clinical trial of microarray technology in children, including infants as young as nine months old.
The study evaluated the safety, immunogenicity, and acceptability of the leading commercially available measles-rubella (MR) vaccine administered via microarray.
Vaccination by microarray was found safe and well tolerated with no allergic reactions or related serious adverse events.
Day-42 immunogenicity showed seroprotection rates for MR in all cohorts for both the microarray (93.2% - 100%) and SC injection (89.8% - 100%) groups.
And in infants who were MR-vaccine naïve at the start of the trial, seroconversion rates were high and similar for both the microarray (92.9% -100%) and SC injection groups (89.7%-100%).
Over 90% of the parents of toddlers and infants enrolled in the trial, which took part in an acceptability survey, said that the microarray technology would be better than SC injection to give vaccines to children.
"Supporting innovations in vaccine delivery is critical to addressing ongoing health inequities," said James Goodson, Senior Scientist and Epidemiologist in the Global Immunization Division at the Centers for Disease Control and Prevention and co-investigator for the study, in a press release on May 17, 2023.
The technology significantly simplifies the transport, storage, and administration of vaccines traditionally delivered via injection and eliminates sharps waste.
The U.S. CDC Advisory Committee on Immunization Practices (ACIP) confirmed it had scheduled a vaccine review meeting open to the public for June 21-23, 2023.
Conducted at the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia, this meeting's purpose is to review scientific data and vote on vaccines and candidate recommendations for Respiratory Syncytial Virus vaccines; Recommendations for adult Polio vaccinations; Flu shots for the 2023-2024 season, Chikungunya, COVID-19, Dengue, Meningococcal, and Mpox vaccines.
This meeting's agenda will be led by Dr. Grace Lee, the ACIP Chair.
ACIP recommendations are public health guidance for the safe use of vaccines and related biological products.
The non-binding recommendations include the age(s) when the vaccine should be given, the number of doses needed, the amount of time between doses, and precautions and contraindications.

Pfizer Inc. today announced that the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted that the available data support the efficacy and safety of its unadjuvanted bivalent respiratory syncytial virus (RSV) prefusion F vaccine candidate RSVpreF or PF-06928316 (ABRYSVO™).
The vaccine candidate is under FDA review for preventing medically attended lower respiratory tract disease (MA-LRTD) and severe MA-LRTD caused by RSV in infants from birth up to six months of age by active immunization of pregnant women.
The VRBPAC voted 14 to 0 on effectiveness and 10 to 4 on safety.
“We are encouraged by the outcome of today’s VRBPAC meeting as it is a critical step forward in the scientific community’s long-sought-after goal to help prevent RSV disease in infants during their most vulnerable first six months of life,” said Annaliesa Anderson, Ph.D., Senior Vice President and Chief Scientific Officer, Vaccine Research and Development, Pfizer, in a related press release.
Additional RSV vaccine and monoclonal antibody news are posted by Precision Vaccinations.

The U.K. Health Security Agency (UKHSA) recently detected influenza A (H5) virus in two poultry workers who have recently worked on an infected poultry farm in England.
Neither person has experienced any avian influenza (bird flu) symptoms, and both have since tested negative.
Professor Susan Hopkins, Chief Medical Advisor at UKHSA, stated in a press release on May 16, 2023, "Current evidence suggests that the avian influenza viruses we're seeing circulating in birds around the world do not spread easily to people."
"However, we know already that the virus can spread to people following close contact with infected birds, and this is why, through screening programs like this one, we are monitoring people who have been exposed to learn more about this risk."
"Globally, there is no evidence of the spread of this strain from person to person, but we know that viruses evolve all the time, and we remain vigilant for any evidence of changing risk to the population."
"It remains critical that people avoid touching sick or dead birds."
In the U.S., one bird flu vaccine is approved by the U.S. FDA.
Precision Vaccinations posts updated news on the global avian influenza outbreak in birds, mammals, and humans.

The U.S. Food and Drug Administration (FDA) today published the Briefing Document for the Vaccines and Related Biological Products Advisory Committee (VRBPAC) review of ABRYSVO™, a respiratory syncytial virus (RSV) vaccine.
Pfizer Inc.'s ABRYSVO is a bivalent vaccine candidate comprised of two preF proteins selected to optimize protection against RSV A and B.
This digital meeting is scheduled for May 18, 2023, and starts at 8:30 AM ET and is open to the public.
The VRBPAC provides independent expert advice to the FDA on broad scientific topics or certain products to help the agency make sound decisions based on the available science.
GSK's AREXVY™ RSV OA single-dose RSV vaccine was previously approved by the FDA for seniors.
Furthermore, there are several other RSV vaccine candidates conducting late-stage studies.
Update May 18, 2023 - EXECUTIVE SUMMARY - This document summarizes the favorable benefit-risk profile for Pfizer’s RSVpreF (Abrysvo), a bivalent respiratory syncytial virus (RSV) stabilized prefusion F subunit vaccine (RSVpreF) for the proposed indication for prevention of lower respiratory tract disease and severe lower respiratory tract disease caused by RSV in infants from birth through 6 months of age, by active immunization of pregnant individuals.

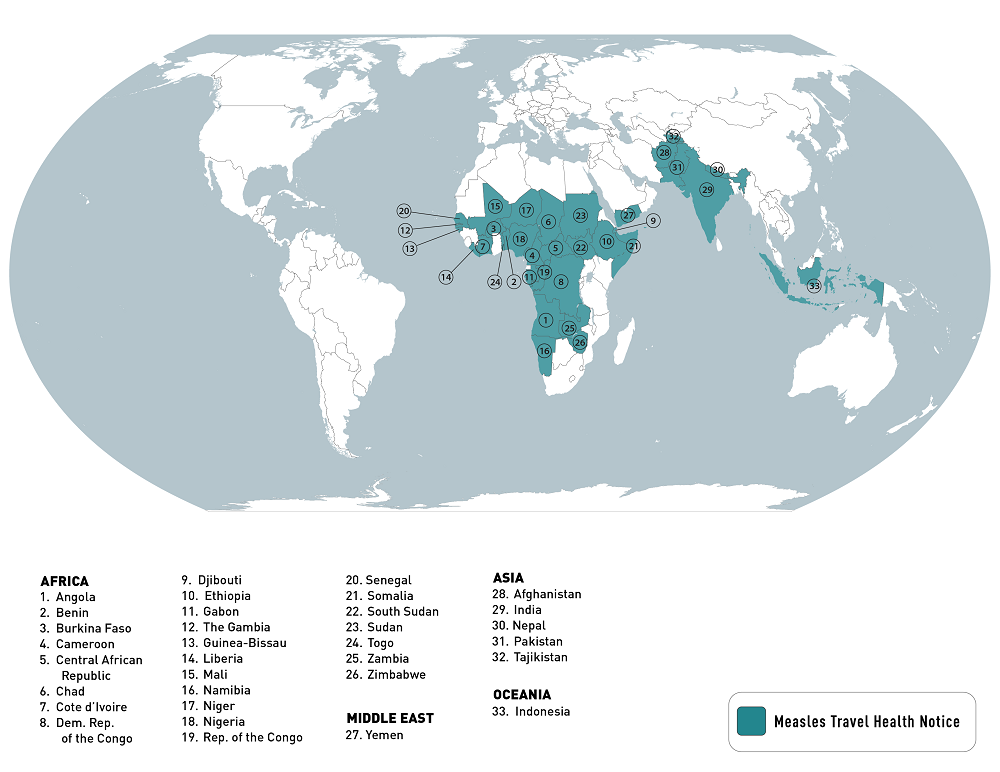
The U.S. Centers for Disease Control and Prevention (CDC) today reissued its Watch - Level 1, Practice Usual Precautions notice regarding the ongoing worldwide measles outbreak.
As of May 16, 2023, the CDC has compiled an extensive list of countries reporting measles outbreaks in 2023.
This list is led by India, with about 68,000 measles cases.
Measles is caused by a highly contagious virus that spreads through the air by direct contact with infectious droplets or by airborne spread when an infected person breathes, coughs, or sneezes.
The measles virus can live for up to two hours in airspace after an infected person leaves an area.
Furthermore, people can spread measles up to four days before and four days after a rash.
While there have only been ten measles cases in the U.S. this year, this virus remains a risk to anyone under-vaccinated.
The CDC says all international travelers, including infants and preschool-aged children, should be protected against measles before traveling abroad.
Various measles vaccines are offered in the U.S. at clinics and community pharmacies.