Search API
Atea Pharmaceuticals, Inc. today announced an achievement from its Hepatitis C Virus (HCV) programs. The Company recently reported positive initial data from the first 52 patients in the lead-in cohort of its Phase 2 combination 8-week study of bemnifosbuvir and ruzasvir (RZR) for the treatment of HCV.
"We are excited to share that the initial data from the Phase 2 combination 8-week study showed a 98% Sustained Virologic Response at Week #4, which exceeds our efficacy criterion of >90% for continuing the study. Based on these data, we plan to imminently reinitiate enrollment to complete the Phase 2 study, and topline results are anticipated in the third quarter of 2024," said Jean-Pierre Sommadossi, Ph.D., Chief Executive Officer and Founder of Atea, on January 8, 2024.
"While direct-acting antivirals have transformed HCV treatment, substantial unmet needs still exist, and the rate of new and reinfection currently exceeds cure rates in the U.S. where 2.4 million individuals are estimated to be infected."
"The key unmet needs identified by healthcare providers in market research recently conducted by Atea include shorter length of treatment with fewer contraindications, particularly drug-drug interactions."
The full, unedited press release is available at this company link.
Hepatitis C is a liver infection caused by HCV that is spread through contact with blood from an infected person.
According to the U.S. CDC, for some people, hepatitis C is a short-term illness, but for more than half of people who become infected with the hepatitis C virus, it becomes a long-term, chronic infection.
Chronic hepatitis C can result in serious, even life-threatening, health problems like cirrhosis and liver cancer.
Most health agencies encourage people at risk to get tested.
The CDC's website confirmed on January 10, 2024, that there is no vaccine for hepatitis C.

In late 2023, the World Health Organization issued its first-ever prequalification approval for a vaccine under its Emergency Use Listing (EUL) regulatory pathway, the novel oral polio vaccine type 2 (nOPV2).
Since this next-generation vaccine rollout began in March 2021, the Global Polio Eradication Initiative (GPEI) reported about 1 billion nOPV2 doses have been administered across 35 countries, protecting children against disease.
WHO prequalification will enable additional countries to access the vaccine in response to outbreaks of type 2 variant poliovirus (cVDPV2).
As of January 3, 2024, the GPEI reported that 325 cases of cVDPV2 had been confirmed in 2023, compared to 689 cases in 2022.
While nOPV2 has played a vital part in this reduction, its success, like any polio vaccine, depends on the ability to implement high-quality immunization campaigns that reach every child rapidly, says the GPEI.
"This is a historic milestone for polio eradication and public health," commented WHO Director-General Dr Tedros Adhanom Ghebreyesus in a press release.
"Novel oral polio vaccine type 2 has blazed a trail for other new vaccines that address critical health emergencies, and its use demonstrates the utility of the EUL mechanism in helping to rapidly get new products to where they're needed most."
The nOPV2 vaccine is genetically more stable than existing oral polio vaccines, with a lower risk of reversion to neurovirulence. In addition, this nOPV2 vaccine produces a gut reaction that stops virus transmission using a more stable version of the OPV, which is much less likely to cause paralysis.
The WHO's EUL is reserved for using yet-to-be-licensed vaccines, medicines, and diagnostic tools during public health emergencies like polio outbreaks.
As of January 10, 2024, the nOPV2 vaccine is unavailable in the United States.
According to a recent U.S. CDC Travel Health Notice, over 30 countries reported polio outbreaks in 2023.

The Philadelphia Health Department reported on January 9, 2024, two additional confirmed measles cases, increasing the total number of confirmed measles cases to eight.
In response, the City is working to identify everyone who may have been exposed, checking their vaccine status, warning them that they may have been exposed, and issuing quarantine and exclusion recommendations where necessary.
Philadelphia has expanded the number of potential measles virus exposure locations and dates to account for what has been learned during the ongoing case investigation.
The Health Department’s measles blog post contains the complete list of locations.
The Health Department continues to offer the measles, mumps, and rubella (MMR) vaccines for free at City Health Centers. And the City is offering walk-in MMR vaccinations at three City health centers for a limited time.
Philadelphia and Jefferson Health initially notified the public of this health risk on December 23, 2023.

HilleVax, Inc. and Chengdu Kanghua Biological Products Co., Ltd. today announced the entry into an exclusive license agreement for rights to Kangh's hexavalent virus-like particle (VLP) vaccine candidate for norovirus.
Referred by HilleVax as HIL-216, outside of Greater China, this VLP targets six of the most common norovirus genotypes, including GI.1, GII.2, GII.3, GII.4, GII.6, and GII.17.
HilleVax stated it intends to launch a Phase 1 trial in 2024.
According to the press release on January 8, 2024, the Investigational New Drug application for HIL-216 was cleared by the U.S. FDA in September 2023.
As of January 9, 2024, the FDA has not approved any norovirus vaccine candidate for use in the U.S.
Rob Hershberg, MD, Ph.D., Chairman and Chief Executive Officer at Hillevax, commented, "Our bivalent norovirus VLP vaccine candidate, HIL-214, remains the most advanced norovirus vaccine candidate in clinical development, and we are on track to report topline safety and efficacy data in mid-2024."
"We believe that HIL-214 will be the first norovirus vaccine submitted for registration and, if approved, would address the significant unmet medical need in infants and other at-risk populations."
"We further believe that HIL-216 is an exciting addition to the HilleVax portfolio as a next-generation, higher valency VLP-based vaccine and is an ideal fit with the expertise, capabilities, and long-term aspirations of HilleVax."
HilleVax confirmed it will pay Kangh an upfront payment of $15 million with the potential for additional payments of up to $255.5 million upon achieving specific development and sales milestones. Kangh can also receive a single-digit tiered royalty on net sales outside of Greater China.
Globally, norovirus is estimated to result in approximately 700 million cases of acute gastroenteritis and 200,000 deaths per year, resulting in over $4 billion in direct health system costs and $60 billion in societal costs per year.
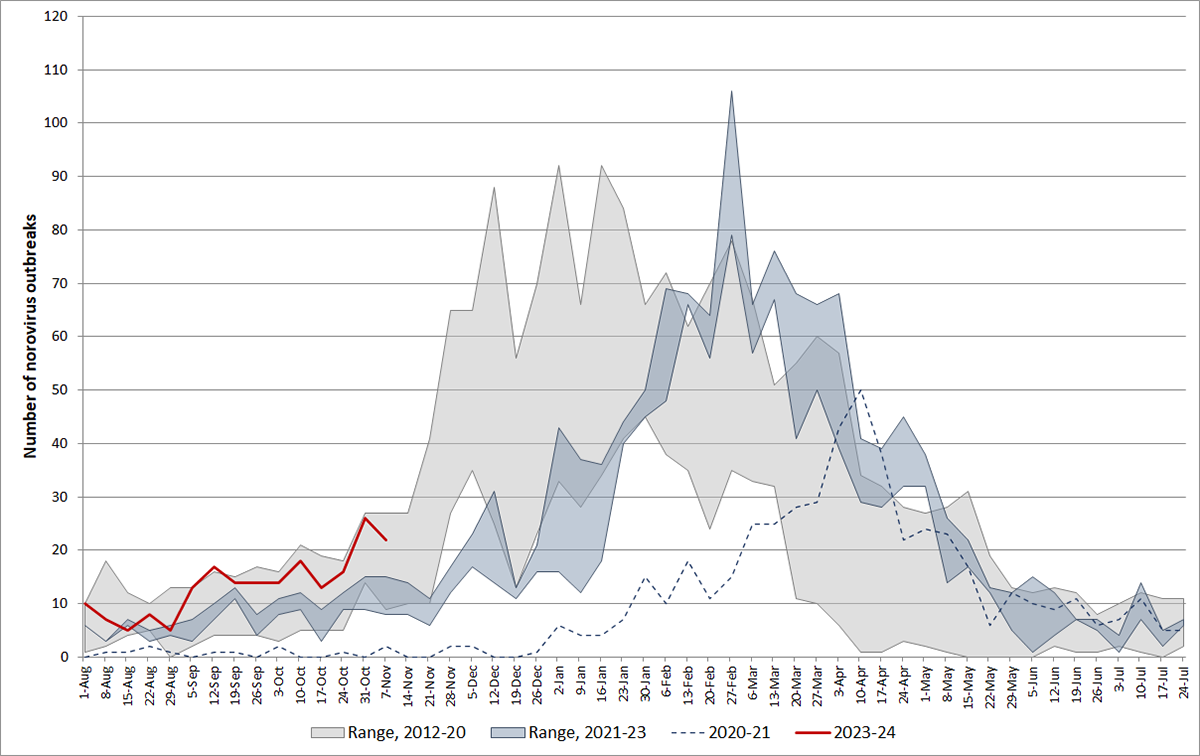
According to the U.S. CDC, norovirus is a contagious virus that causes vomiting and diarrhea. Anyone can get infected and sick with norovirus. Most norovirus outbreaks in the U.S. happen from November to April.
From August through November 13, 2023, there were 202 norovirus outbreaks reported by NoroSTAT-participating states. During the same period last season, 134 norovirus outbreaks were reported by these states, according to the CDC.
HilleVax is a clinical-stage biopharmaceutical company based in Boston, MA, focused on developing and commercializing novel vaccines.
BioArctic AB's partner Eisai announced today that Leqembi® (lecanemab-irmb) has been approved in China as a treatment for mild cognitive impairment (MCI) due to Alzheimer's disease (AD) and mild AD dementia.
China is the third country to grant marketing approval, following the traditional approval in the United States in July 2023 and Japanese approval in September 2023.
Eisai's preparations for the Chinese launch in the third quarter of 2024 are underway.
Eisai estimates that there are 17 million patients with MCI or mild dementia due to AD in China today, which is expected to increase with the aging of the population. Eisai will distribute the product in China and conduct information provision activities through specialized Medical Representatives.
In addition, BioArctic has the right to commercialize lecanemab in the Nordic region, pending European approval, and Eisai and BioArctic are currently preparing for joint commercialization in the region.

Transgene and NEC Corporation today announced the signing of a further development collaboration agreement to continue the clinical evaluation of the individualized neoantigen cancer vaccine TG4050.
TG4050 is currently being evaluated in a randomized multicenter Phase I trial as a single agent in the adjuvant treatment of HPV-negative head and neck cancers. Based on promising data obtained in this Phase I trial, Transgene and NEC are preparing a randomized Phase I/II extension of this trial slated to start in 2024.
This new trial builds on compelling first signs of efficacy and induction of specific T-cell responses to generate a comprehensive set of immunological and clinical data to demonstrate the potential of TG4050 further.
Additional immunological and clinical data from the Phase I trial is expected at a scientific conference in the first half of 2024.
Alessandro Riva, Chairman and CEO of Transgene, commented in a press release, "We are pleased to announce the extension of our agreement with NEC, which marks a significant milestone in our collaboration."
"We look forward to continuing to treat patients with our individualized cancer vaccine TG4050. The compelling initial Phase I data presented with NEC at ASCO 2023 showed that all evaluable patients treated with TG4050 monotherapy developed a specific immune response and remained disease-free."
"Our joint clinical development plan builds on these promising data in a setting where there is no approved treatment to prevent patient relapse after adjuvant chemoradiotherapy."
"We believe that TG4050, by combining a powerful and immunogenic viral vector with an extremely sophisticated neoantigen selection tool, has the potential to address major medical needs in the adjuvant treatment of solid tumors."
TG4050 is based on Transgene's viral vector-based myvac® platform and powered by NEC's cutting-edge AI capabilities for identifying and predicting the most immunogenic neoantigens.
A U.S. CDC-funded Original Research study concluded that two doses of recombinant zoster vaccine (RZV) were highly effective, although less effective against Herpes Zoster (shingles) than in the previous clinical trials.
Published by the Annals of Internal Medicine on January 9, 2024, this Real-World Setting study included nearly 2 million persons who contributed 7.6 million person-years of follow-up.
After adjustment, the vaccine efficacy (VE) of 1 dose was 64%, and VE of 2 doses was 76%.
After one dose only, VE was 70% during the first year, 45% during the second year, 48% during the third year, and 52% after the third year.
After two doses, VE was 79% during the first year, 75% during the second year, and 73% during the third and fourth years.
These findings underscore the importance of the second vaccine dose, wrote these researchers.
Currently, the CDC recommends the Shingrix® vaccine to prevent herpes zoster and related complications for most adults aged 50 and older.
There is no specific time you need to wait before administering Shingrix to patients who have had herpes zoster.
However, it would be best if you did not give Shingrix to patients who are experiencing an acute episode of herpes zoster or a pregnant woman.

According to a Medical News Brief by Emily Harris with The JAMA Network, observational data from more than 1.6 million people in the United States suggests that a high-dose vaccine may also be more effective than standard-dose vaccines for certain adults.
Reported in the New England Journal of Medicine in December 2023, the Original Article evaluated a recombinant vaccine that contains more than triple the amount of influenza hemagglutinin protein compared with standard-dose vaccines.
Flu cases were about 15% lower for people aged 50 to 64 who received the recombinant vaccine.
Because standard-dose vaccines only prevent up to 40% to 60% of influenza cases, decreasing the number of cases by an additional 15% would still “provide a substantial public health benefit, especially during more severe influenza seasons,” the researchers wrote.
As of December 31, 2023, the U.S. CDC reported that over 155 million egg, cell, and nasal-based influenza vaccines, which are generally available at local pharmacies, had been distributed this flu season.