Search API
The journal Vaccine recently published a manuscript entitled "Thermostable bivalent filovirus vaccine protects against severe and lethal Sudan ebolavirus and marburgvirus infection."
This publication describes the preclinical efficacy of a novel, single-vial, bivalent thermostabilized vaccine providing 100% protection in the most rigorous non-human primate challenge models against Sudan ebolavirus (SUDV) and Marburg marburgvirus (MARV) infections.
Recent outbreaks have occurred in Africa, with increased frequency in 2023.
There are currently no approved vaccines or therapeutics for either SUDV or MARV infections.
However, vaccines are available for Zaire ebolavirus (EBOV) infections in 2024, but they provide no protection against SUDV or MARV infection.
"Filoviruses such as EBOV, SUDV, and MARV are some of the most lethal viruses known, and they are endemic in areas of the world where the power supply and distribution network can be uncertain, says the World Health Organization.
A thermostabilized vaccine in a single vial format would significantly enhance any public health response to a new outbreak, at its source," stated Axel Lehrer, Ph.D., Associate Professor, Department of Tropical Medicine, Medical Microbiology and Pharmacology, University of Hawaiʻi at Mānoa, in a press release.
"Our work to date has demonstrated the feasibility of rapid and efficient manufacturing, as well as the ability to thermostabilize multiple antigens that can then be stored for extended times at temperatures exceeding 100 degrees Fahrenheit."
"The use of a bivalent vaccine has the potential to both prevent future infections with these pathogens and potentially mitigate future outbreak events, potentially using an accelerated dosing regimen."
The thermostabilized filovirus vaccine program continues to advance with the support of a National Institute of Health grant and a Small Business Innovation Research grant awarded to Soligenix, Inc.

Inventprise Inc. today announced the completion of vaccination in their Phase 2 dose-ranging study of its 25-valent pneumococcal conjugate vaccine (IVT PCV-25) in young adults.
On January 2, 2023, the company stated that this Phase 2 study is essential in developing an affordable, expanded-coverage pneumococcal conjugate vaccine (PCV). This vaccine candidate is designed to help prevent pneumococcal disease caused by serotypes not covered in the current PCVs.
“PCVs are the world’s most complex vaccines, and increasing the number of serotypes (25) and manufacturing capacity has been challenging since conjugate vaccines were first developed,” commented Yves Leurquin, President & CEO of Inventprise, in a press release.
As of 2024, several approved PCVs and vaccine candidates are conducting research. However, some do not include critical disease-causing serotypes.
“This study was designed to evaluate safety and antibody responses following single doses of three different formulations of IVT PCV-25 in young adults to pave the way for future studies in infants and older adults. In Canada, as well as elsewhere in the world, there continues to be significant rates of disease due to serotypes not covered in licensed vaccines so we are very interested in the potential for broader coverage,” says Dr. Joanne Langley, the study’s principal investigator at the Canadian Center for Vaccinology.
As a leading cause of deadly childhood pneumonia, sepsis, meningitis, and debilitating middle-ear infections, the pneumococcus bacterium is responsible for an estimated 300,000 deaths per year in children less than five years of age worldwide.
Inventprise is producing the IVT PCV-25 vaccine at its automated manufacturing facilities in Washington State. Funding for the development of IVT PCV-25 has been achieved with support from the Bill & Melinda Gates Foundation.

The Alaska Department of Environmental Conservation recently reported a polar bear was found dead after being infected with the highly pathogenic avian influenza (HAPI).
The polar bear was found on October 1, 2023, near Utqiagvik, a North Slope community in the United States. Following HAPI sample testing, the bear's death was confirmed on December 6, 2023.
This was also the first Endangered Species Act-listed animal in Alaska known to fall victim to HAPI.
There has been a global increase in HPAI outbreaks due to the genetic diversity of circulating virus strains, which rapidly spread in birds.
This World Organisation for Animal Health report issued in October 2023 provides an update on the HPAI situation.
Since people can become infected by the HAPI virus, the U.S. government has been preparing vaccines for an outbreak.
According to the Centers for Disease Control and Prevention, about 20 million H5N1 and 12 million H7N9 vaccines were available in the U.S. National Strategic Stockpile in 2023.

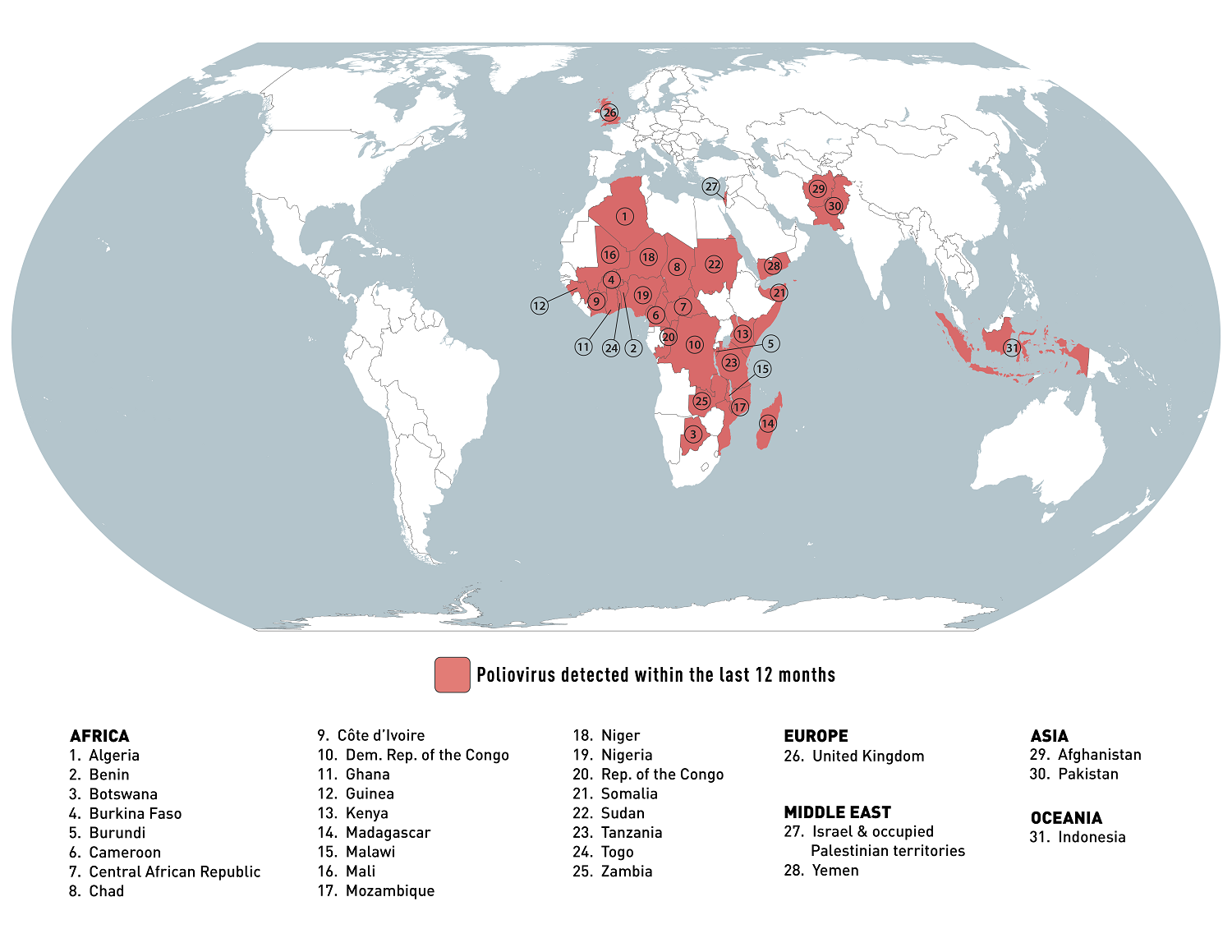
The last report of the Polio Eradication Initiative (GPEI) confirmed additional vaccine-derived poliovirus type 2 (cVDPV2) cases.
The west African country of the Islamic Republic of Mauritania confirmed its first cVDPV2 case in Nouakchott Nord, the first one in 2023.
And Indonesia's confirmed one cVDPV2 case was reported this week in Jawa Tengah. There have been four cVDPV2 cases this year and one case in 2022.
Indonesia Ministry of Health previously completed two rounds of immunization for children below five years of age with the nOPV2 vaccine, regardless of their prior vaccination status. The first round was implemented in April 2023.
The nOPV2 vaccine is a modified version of the type 2 monovalent OPV and is genetically more stable.
The administrative coverage of the bivalent oral polio vaccine between 2018 and 2022 in West Java ranged from 88% to 102%, while coverage of inactivated polio vaccine ranged from 26% to 106%.
Vaccine-derived poliovirus is a strain mutated from the strain initially contained in the oral polio vaccine (OPV).
OPV vaccines contain a live, weakened form of poliovirus that replicates in the intestine for a limited period, thereby developing immunity by building up antibodies, says the World Health Organization (WHO).
On rare occasions, when replicating in the gastrointestinal tract, OPV strains genetically change and may spread in communities not fully vaccinated against polio.
According to the GPEI's data, as of December 31, 2023, about 1 billion nOPV2 vaccinations have been completed globally.
WHO's International Travel recommendations include .... that all travelers to the 35 polio-affected areas should be fully vaccinated against polio.
Novotech recently released the latest global clinical trial landscape report on HIV, a condition now affecting more than 39 million people.
According to the HIV Global Clinical Trial Landscape report published on December 18, 2023, the biopharma industry has initiated over 1,000 HIV clinical trials worldwide since 2018.
The distribution of the trials is as follows: Asia-Pacific accounts for 29% of trials, Europe at 28%, and North America at 26%, while the Rest of the World contributes a moderate share of 17%.
The report delves into the multifaceted landscape of HIV, focusing on its impact, treatment, and global initiatives.
It begins by elucidating HIV's progression, from its attack on the immune system to the potential development of AIDS. In the United States, there were 1.2 million HIV cases, leading to 19,986 deaths in 2022.
At the report's core lies the worldwide strategy articulated by organizations such as WHO, the Global Fund, and UNAIDS to eradicate the HIV epidemic by 2030.
Moreover, the report explores cutting-edge research, including long-acting injectables, HIV vaccines, and gene editing, underscoring the potential to find an HIV cure.
As of December 31, 2023, there are no approved HIV vaccines available, but several vaccine candidates are conducting studies.
Novotech's research analyst team provides these expert reports every month, free of charge.

Mexico's president recently announced a "super pharmacy" that will help patients nationwide access medicines and vaccines.
According to VOA reporting on December 29, 2023, President Andrés Manuel López Obrador's solution is a centralized warehouse on the outskirts of Mexico City intended to complement local health facilities.
If a patient can't get needed medications at a local hospital, the patient's doctor or pharmacist could have it delivered from the 40,000-square-meter warehouse.
"The pharmacy is going to be big, big, big, and it is going to have all the medications used in the health system," López Obrador said.
The question is whether Mexico can overcome its history of being bad at regulating the pharmaceutical industry, buying medicines, storing them, and distributing them. Extreme centralization also hasn't helped Mexico much in the past in many areas, wrote VOA/AP.
The U.S. Centers for Disease Control and Prevention (CDC) recently updated its Mexico Yellow Book for 2024, identifying numerous endemic diseases such as dengue, varicella, and measles.
And the CDC issues Travel Health Alerts regarding specific disease outbreaks in Mexico and advises that all travelers should be updated on their routine immunizations and travel vaccinations.

Thailand is a geographically diverse country, a little smaller than Texas, and a trendy destination for millions of tourists.
As New Year's Eve 2023 approaches, the U.S. Centers for Disease Control and Prevention (CDC) recently stated transmission of the Zika virus is a health risk in Thailand.
And the Hong Kong Centre for Health Protection (CHP) of the Department of Health confirmed it is aware of the recent Zika outbreaks in Thailand.
According to Thailand's Bureau of Epidemiology, 742 Zika cases have been recorded in 2023, which is much higher than in the past three years.
Among the recent Zika cases, 160 were recorded in Bangkok, which has about 11 million residents.
Given the Zika cases in Thailand this year, the CHP specifically reminded members of the public, especially pregnant women and women preparing for pregnancy, that they must adopt necessary precautions against mosquito-borne diseases.
Furthermore, the U.S. Embassy in Thailand recently informed U.S. citizens that the CDC says that if you decide to travel, prevent mosquito bites and sexual exposure to Zika during and after travel, and wait two months after returning before becoming pregnant.
From a prevention perspective, there are no approved Zika vaccines as of December 30, 2023. However, several vaccine candidates are conducting clinical research.
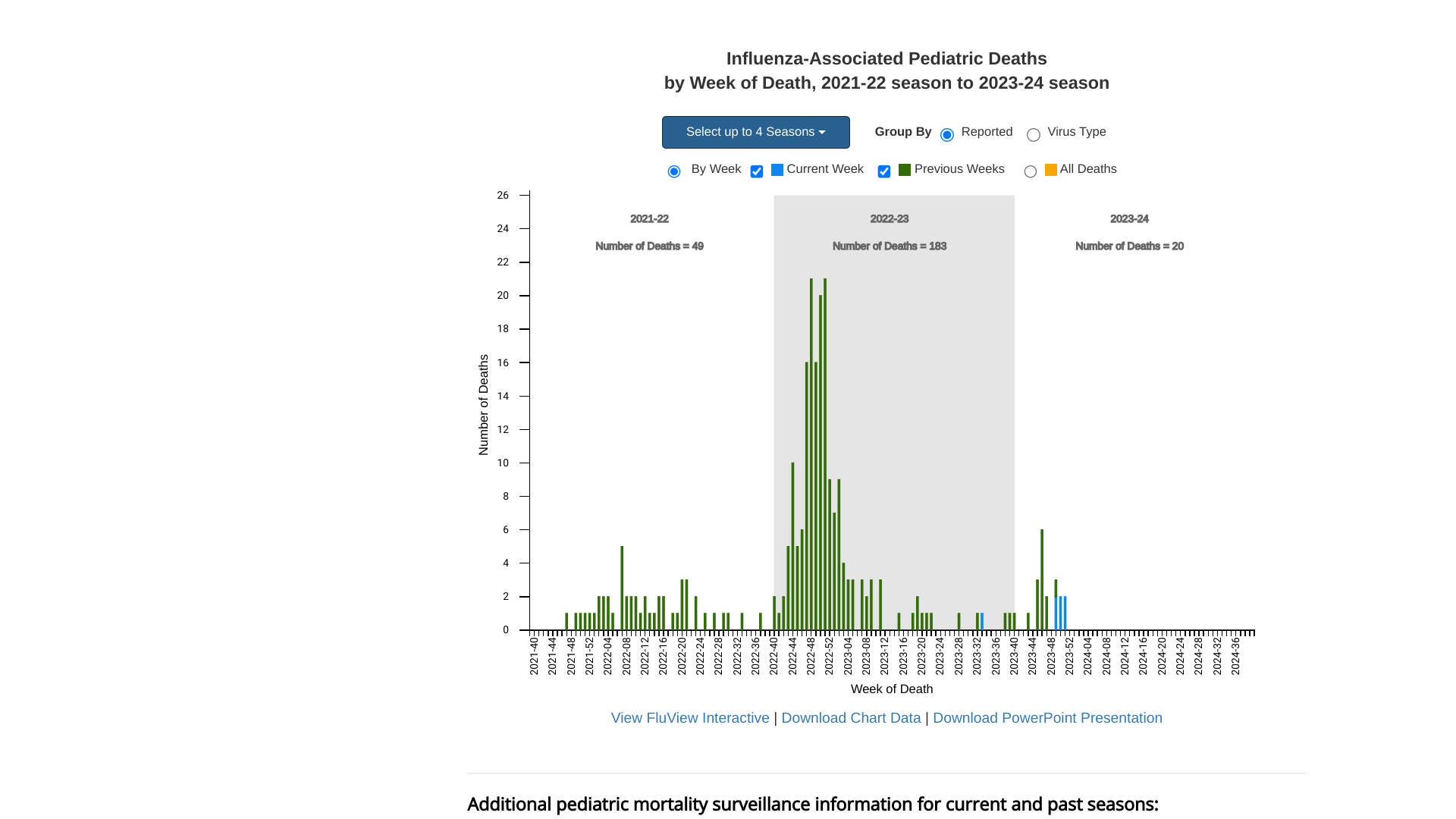
As the 2023 flu season draws to a close, the U.S. Centers for Disease Control and Prevention (CDC) announced today that seasonal influenza activity is elevated and above baseline in all 10 HHS Regions.
And outpatient respiratory illness is above baseline nationally for the eighth consecutive week.
As of December 29, 2023, the CDC confirmed a total of 20 pediatric deaths have occurred during the 2023-2024 season.
Last flu season, the CDC reported 183 pediatric deaths.
The CDC also estimated that there have been at least a total of 4,500 deaths from flu so far this season.
To reduce the health risks from the flu, the CDC recommends that everyone six months and older get an annual flu vaccine as long as influenza viruses are spreading.
Vaccination in late December 2023 can still provide benefits into 2024.
The good news is that over 155 million flu shots have been distributed in the U.S. this season. These egg, cell, and nasal-based flu shots remain available at most pharmacies.
Jiangsu Recbio Technology Co., Ltd. today announced that the novel adjuvanted recombinant shingles vaccine candidate REC610 recently achieved positive results in the interim analysis of the first-in-human clinical trial in the Philippines.
Published on December 29, 2023, the Interim Analysis results showed that REC610 demonstrated an overall favorable safety and tolerability profile in healthy participants aged 40 and above after two vaccination doses.
REC610 induced strong gE-specific humoral and cellular immune responses, which were evident after the first vaccination and peaked 30 days after the second vaccination.
The humoral and cellular immune responses were comparable between REC610 and the Shingrix® vaccine comparison group, and the immune response level in the REC610 group was numerically higher than that in the Shingrix group.
REC610 is intended to prevent shingles in adults aged 40 and above. It is equipped with a novel adjuvant BFA01 independently developed by the Company, which can promote the production of high levels of VZV glycoprotein E (gE)-specific CD4+ T cells and antibodies.
REC610 received a drug clinical trial approval notice issued by the National Medical Products Administration in October 2023. It is approved as a preventive 3.3 biological product in its Phase I and III clinical trials in China.
The Company will soon adopt a randomized, double-blind, parallel controlled phase I clinical trial in 180 healthy adult subjects aged 40 and above in Mainland China to evaluate the safety, tolerability, and immunogenicity of REC610.
According to statistics, China's population aged 40 and above is approximately 700 million, and about 6 million new shingles cases occur each year in China.
Furthermore, the incidence of shingles has gradually become younger in recent years.
Only Shingrix is on the market in China, and there is a strong demand for import substitution, according to Jiangsu Recbio Technology's press release.
Beginning in January 2024, Chongqing Zhifei Biological Products will have exclusive rights to import and distribute Shingrix in China.