Search API
According to recent reports out of the states of Pennsylvania and Washington, measles outbreaks have been confirmed in potentially unvaccinated people in 2024.
On January 12, 2024, the Philadelphia Health Department confirmed eight measles cases spanning 2023 and 2024.
Health agencies alerted healthcare providers in the northwest after six measles cases were identified in Clark and Wahkiakum counties in Washington.
The U.S. Centers for Disease Control and Prevention (CDC) says that since the United States has such a high measles vaccination rate, most measles cases are related to international travelers.
Last year, ten countries reported over 90% of all measles cases worldwide, led by Yemen and India.
During 2023, a total of 48 measles cases were reported by twenty U.S. jurisdictions. The CDC did not disclose the vaccination status of the measles cases.
The good news from the CDC is that various measles vaccines (MMR) are available in 2024, generally available at clinics and pharmacies in the U.S.
Furthermore, before heading abroad in 2024, the CDC publishes this digital app to help travelers determine whether or not they need MMR vaccination before departure.
As the influenza and Respiratory syncytial virus (RSV) seasons diminish in the U.S., world leaders express their disappointment with respiratory disease vaccination rates.
"Too many people are in need of serious medical care for flu, for COVID, when we can prevent it," said Maria Van Kerkhove, the World Health Organization's interim director of epidemic and pandemic preparedness, as reported by Reuters on January 12, 204.
Kerhove cited "incredibly low" vaccination rates against flu and COVID-19 in many countries this season.
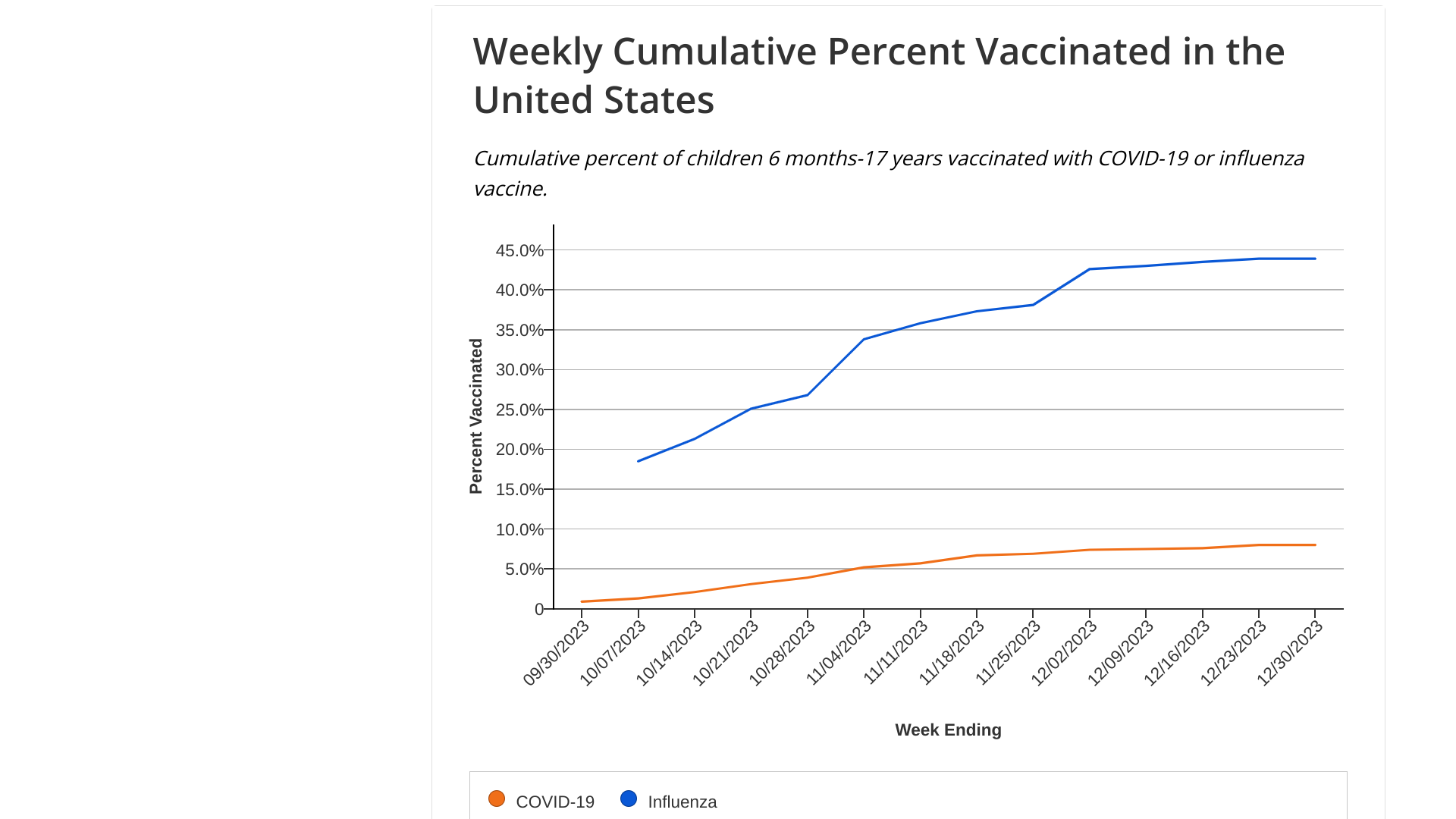
According to the U.S. Centers for Disease Control and Prevention's (CDC) latest data, the percentage of the population reporting receipt of COVID-19, influenza, and RSV vaccines remains low for adults.
- The updated 2023-24 COVID-19 vaccine is 21.4% for adults 18+, including 41.5% for adults 65+.
- Influenza vaccination is 46.8% for adults 18+, including 74.1% among adults 65+.
- RSV vaccinations for adults over 60 years of age is 20.1%.
As of January 12, 2024, the CDC recommends that all six months and older stay current on COVID-19 and receive a seasonal flu vaccine.
Furthermore, if you are 60 years and older, talk to a healthcare provider to see if RSV vaccination is proper for you this season.
These reportatory vaccines are generally available at U.S.-based pharmacies.
For new mothers, the extended half-life monoclonal antibody Beyfortus™ offers passive immunization for infants and young children to prevent lower respiratory tract infections caused by RSV.
Beyfortus was recently approved for use in China, and the manufacturers have committed to deliver significant quantities to the U.S. in 2024.
In response to an active polio outbreak, the Indonesian government has recently requested the WHO Director-General’s approval to release the novel oral polio vaccine type 2 (nOPV2).
As of January 11, 2024, the WHO issued a Disease Outbreak News alert (DON500) confirming the approval of more than 20 million doses of nOPV2 for two rounds of supplementary immunization activities scheduled for January 15, 2024, and February 19, 2024.
Previously, Indonesia reported four cases of circulating vaccine-derived poliovirus type 2 (cVDPV2) from October 2022 to February 2023.
In late December 2023, the Indonesian Ministry of Health notified WHO of two new confirmed cases of cVDPV2.
Genetic sequencing of isolates at the BioFarma National Polio Laboratory indicates cVDPV2 with 36 nucleotide changes, genetically linked to a case reported in West Java province to the WHO in March 2023.
Vaccine-derived poliovirus is a well-documented strain that has mutated from the strain initially used in oral polio vaccines.
In sporadic instances, the vaccine-derived virus can genetically mutate into a form that can cause paralysis, just as the wild poliovirus does – this is what is known as a vaccine-derived poliovirus (VDPV).
The detection of VDPV in at least two different sources, at least two months apart, that are genetically linked and showing evidence of transmission in the community is classified as cVDPV.
According to the WHO, the overall risk is assessed as high at the national level. At the regional level, the overall risk is assessed to be moderate.
Furthermore, the WHO advises against implementing any travel or trade restrictions on Indonesia based on the current information available on this event.
As of January 12, 2024, about 1 billion doses of the nOPV2 vaccines have been distributed.
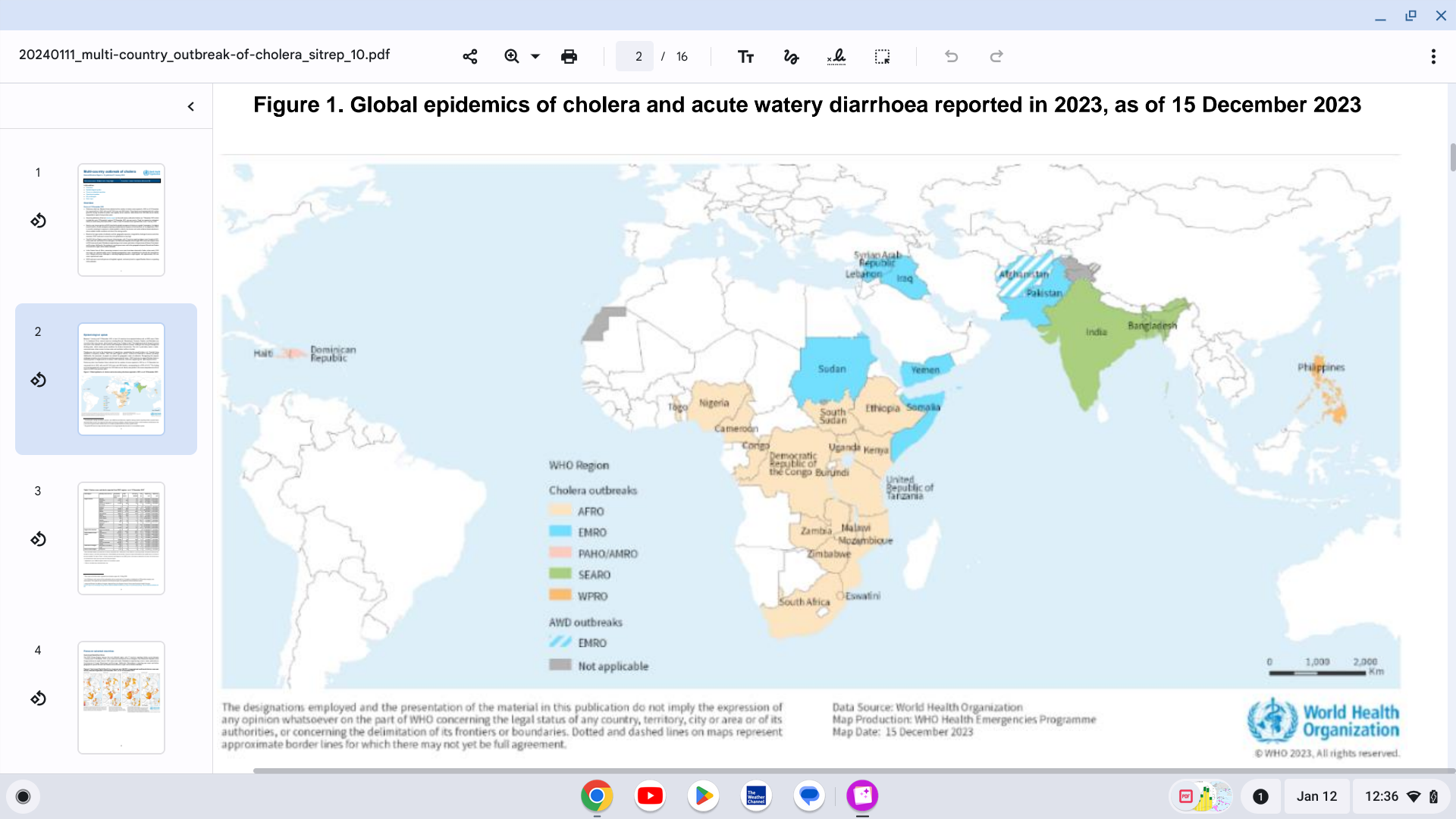
Preliminary data from World Health Organization (WHO) Member States indicate that the number of cholera cases reported in 2023 has surpassed 2022, with over 667,000 cases and 4000 deaths.
In total, at least 30 countries have reported cholera outbreaks in 2023.
As of January 11, 2024, nearly a year has passed since the WHO classified the global resurgence of cholera as a grade 3 emergency.
Based on the large number of outbreaks and their geographic expansion, alongside the shortage of cholera vaccines and other resources, the WHO continues to assess the risk at a global level as very high.
The U.S. Food and Drug Administration, the European Medicine Agency, and the U.K. NHS recommend oral cholera vaccines (OCV) for specific conditions in countries that are undergoing outbreaks.
During 2023, around 65 million OCV doses were requested, with 45% being approved and allocated to 12 countries,
There are three WHO pre-qualified OCVs: Dukoral®, Shanchol™, and Euvichol®.
In August 2023, the U.S. Centers for Disease Control and Prevention (CDC) published Cholera Vaccine: Recommendations, highlighting CVD 103-HgR (Vaxchora®) for travelers ages 2–64 years old going to areas of active toxigenic Vibrio cholerae O1 transmission.
Cholera is an acute intestinal infection that spreads through food and water contaminated with the bacterium Vibrio cholerae, often from feces. Cholera can kill people within hours when not treated, but immediate access to treatment saves lives, says the WHO.
According to the U.S. Centers for Disease Control and Prevention (CDC) latest FluView report, the current flu season in the United States may be winding down.
As of January 12, 2024, the CDC stated that a week of decreased data has been noted after several weeks of increased key flu indicators.
Nationwide, 5.7% of patient visits reported through ILINet during week #1 of 2024 were due to respiratory illness that included fever plus a cough or sore throat. This has decreased compared to Week #52 of 2023.
Based on mortality surveillance data available on January 11, 2024, 1.3% of the deaths during the week ending January 6, 2024 (Week #1) were due to influenza. This percentage increased (≥ 0.1 percentage point change) compared to Week #52.
However, seasonal influenza activity remains elevated in most parts of the country.
And the CDC will continue monitoring the data for an indication of a second period of increased influenza activity that often occurs after the winter holidays.
Should there be a rekindling of influenza activity, ample flu shots should be available at most pharmacies in the U.S. The CDC says vaccination now can still provide benefits this flu season.
The CDC reported that over 155 million influenza vaccines had been distributed last year.
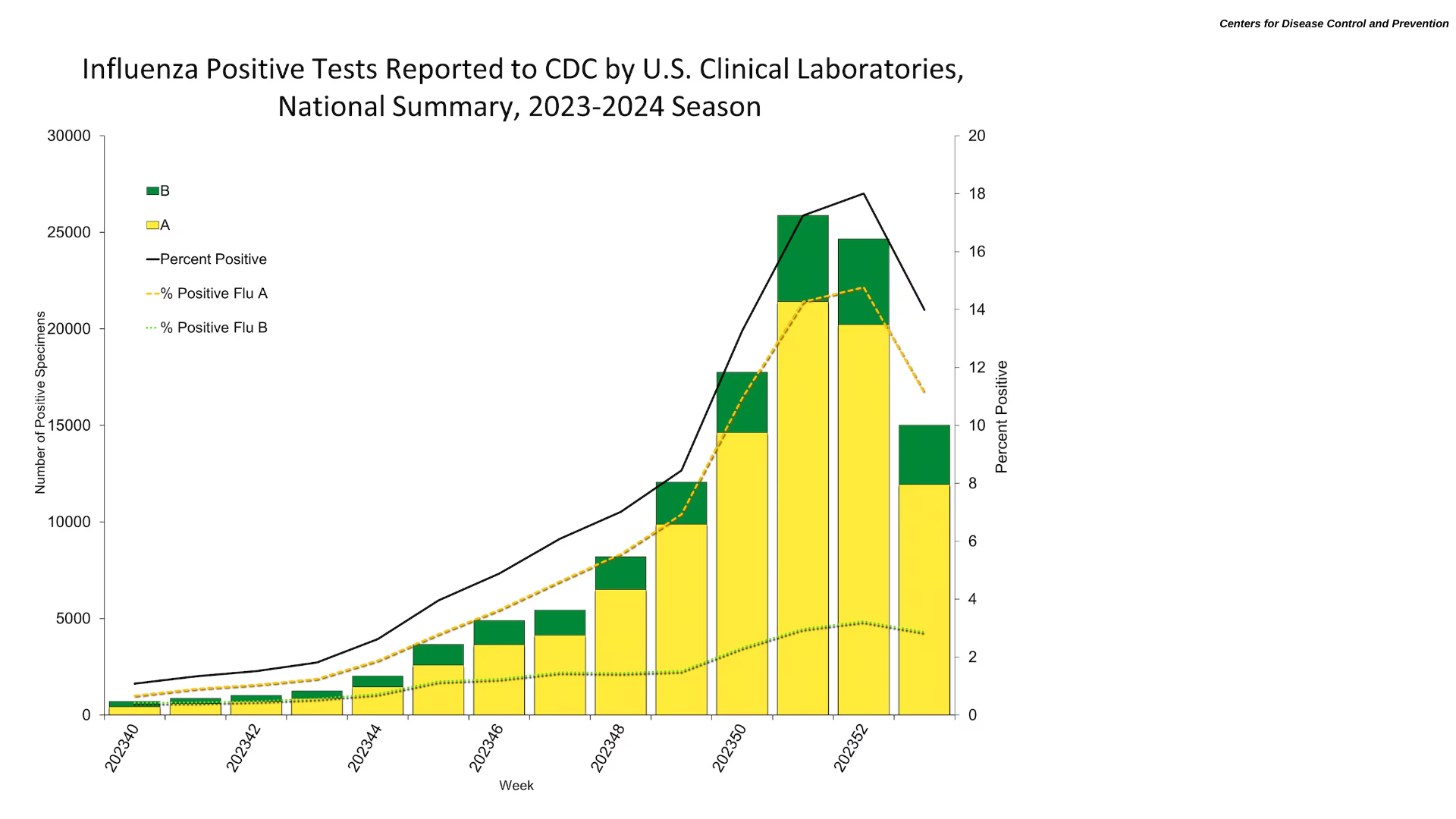
Since Zika outbreaks peaked in 2017, the circulation of this mosquito-borne virus has been confirmed in 89 countries worldwide. Although incidence levels remain low, sporadic increases in Zika cases have been observed in some countries in the Region of the Americas.
The Zika virus is a flavivirus transmitted by mosquitoes of the Aedes genus, which are common in the Americas.
"Most infections with this virus are asymptomatic or mild, making their detection by healthcare systems quite challenging," said María Van Kerkhove, Head of the Emerging Diseases and Zoonoses Unit at the World Health Organization (WHO), in a September 2023 press release.
However, the Pan American Health Organization confirmed that the Federative Republic of Brazi confirmed 33,386 Zika cases last year.
And with climate change impacting the range of disease-carrying mosquitos, several countries in Central America reported Zika outbreaks as of week #52.
- Belize - 281
- Guatemala - 112
- El Salvador - 110
- Mexico - 29
But this trend has yet to progress north of Mexico and the Rio Grande River, as the state of Texas reported (0) Zika cases in 2023.
With regard to complications from this disease, WHO says many people infected with the Zika virus won't have symptoms or will only have mild symptoms.
Unfortunately, pregnant women are particularly susceptible to its effects since it can lead to congenital malformations, such as microcephaly, as well as an increased likelihood of preterm births or spontaneous abortions.
As of January 11, 2024, there are no U.S. FDA-approved Zika vaccines, but several vaccine candidates are conducting clinical research.
According to a letter by a France-based penicillin producer, the U.S. Food and Drug Administration (FDA) has authorized the importation of an injectable drug to treat syphilis.
A letter dated November 21, 2023, Paris-based from Laboratoires Delbert stated: Temporary Importation of Extencilline (benzathine benzylpenicillin) Powder and diluent for reconstitution for injection, 1,200,000 units and 2,400,000 units with Foreign, non-U.S. Labeling to Address Supply Shortage.
Extencilline will be available in the U.S. only by prescription in 2024.
The FDA's website confirmed this authorization on January 10, 2024.
This change in FDA policy is related to the current penicillin supplier having an available disruption.
On June 12, 2023, Pfizer notified the FDA that ll Bicillin ® C-R (penicillin G benzathine and penicillin G procaine injectable suspension) are estimated to deplete in Q3 2023 and Bicillin® L-A (penicillin G benzathine injectable suspension) Pediatric (600,000 units/mL) Prefilled Syringes are estimated to deplete by the end of Q2 2023. Furthermore, these penicillin presentations will not be available until further notice.
Key differences between Bicillin® L-A and Extencilline are available at this link.
Neither penicillin injectable drug is a U.S. FDA-approved syphilis vaccine.
The continued prevalence of Syphilis and Congenital Syphilis in the United States highlights the need for a syphilis preventive vaccine, says the World Health Organization.

Emergent BioSolutions Inc. today announced that it has secured an indefinite-delivery, indefinite-quantity (IDIQ) procurement contract with a maximum value of up to $235.8 million to supply BioThrax® (Anthrax Vaccine Adsorbed) for use by all branches of the United States military as Pre-Exposure Prophylaxis (PrEP) for anthrax disease.
The new contract with the U.S. Department of Defense (DoD), led by the Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense, is comprised of a five-year base agreement ending on September 30, 2028, and an additional five-year option that would extend the contract to September 30, 2033.
Under the initial five-year IDIQ contract, there is a guaranteed purchase minimum of $20.1 million, with future orders estimated at least $20 million each following year.
"As a part of our mission to protect and enhance lives, Emergent is proud to continue supporting and preparing our nation's service members who have a high risk of exposure to anthrax bacteria by supplying BioThrax vaccine," said Paul Williams, senior vice president, products head at Emergent, in a press release on January 11, 2024.
Emergent's BioThrax vaccine is indicated for active immunization to prevent disease caused by Bacillus anthracis in persons 18 through 65 years of age.
BioThrax is also approved for pre-exposure prophylaxis of disease in persons at high risk of exposure.
Furthermore, BioThrax is approved for post-exposure prophylaxis of disease following suspected or confirmed Bacillus anthracis exposure when administered in conjunction with recommended antibacterial drugs.
Biothrax's initial approval in the U.S. was issued in 1970. The U.S. FDA updated STN: BL 103821 in July 2023.

The Oxford Vaccine Group today announced it is leading the first-in-human phase 1 clinical trial of the ChAdOx1 NipahB vaccine candidate.
Funded by the Coalition for Epidemic Preparedness Innovations (CEPI), this vaccine candidate is being developed by researchers at the University of Oxford's Pandemic Sciences Institute.
Fifty-one adults aged 18 to 55 will participate in the trial, which is expected to last 18 months (March 2025), with additional trials expected to follow in a Nipah-affected country, such as Singapore, Malaysia, Bangladesh, or India.
The primary contact of the study (ISRCTN87634044) is Miss Ella Morey, [email protected].
This new study is essential as there are no approved Nipah vaccines to protect people from this disease, which is fatal in around 75% of cases.
Professor Brian Angus, the trial's Principal Investigator and Professor of Infectious Diseases at the Centre for Clinical Tropical Medicine and Global Health commented in a press release on January 11, 2024, "Nipah virus was first identified in 1998, and yet 25 years on the global health community still has no approved vaccines or treatments for this devastating disease."
"Due to the high mortality rate and the nature of Nipah virus transmission, the disease is identified as a priority pandemic pathogen. This vaccine trial is an important milestone in identifying a solution to prevent local outbreaks while helping the world prepare for a future global pandemic."
The World Health Organization (WHO) recognizes Nipah as a priority disease requiring urgent research and belongs to the same family of paramyxoviruses as more well-known pathogens like measles.
According to the WHO, fruit bats carry the disease and may also be transmitted by contact with infected animals (pigs) or from person to person via close contact.
Initially announced in 2023, the Republic of Cameroon intends to launch a malaria vaccine campaign in late January 2024. Cameroon took delivery of 331,200 doses of malaria vaccine in November last year.
According to Manaouda Malachie, Cameroon's minister of public health, this vaccination effort is part of an Africa-wide effort to reduce morbidity and mortality associated with the mosquito-transmitted disease.
Malaria is responsible for about 70% of deaths among children in Cameroon, which has a life expectancy at birth of about 57 years.
"The selected vaccine, Mosquirix™, has been chosen by the country based on its pre-qualification, ensuring guaranteed quality, efficacy, and safety for its inclusion in the vaccination programs," as reported by CamarronOnline.org on January 10, 2024.
Malaria vaccines have been approved for use in Africa and are reported to be effective at preventing disease.
Since 2021, the World Health Organization and the European Medicines Agency have recommended the Mosquirix (RTS,S/AS01) malaria vaccine.
In late 2023, the R21 / Matrix-M™ vaccine was WHO-approved for use in certain countries.
As of January 10, 2024, the U.S. FDA had not approved a malaria vaccine.
