Search API
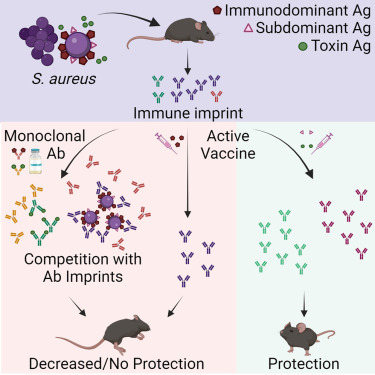
In a new study published in Cell Reports Medicine, researchers at the University of California San Diego School of Medicine explained why people would benefit from a saphylococcus aureus (SA) vaccine.
While SA causes many dangerous health complications, including wound and bloodstream infections, the bacterium is also a normal part of the healthy human microbiome, where it lives peacefully in the nose and on the skin.
These researchers tested a new hypothesis that SA bacteria can trick the body into releasing non-protective antibodies when they first colonize or infect humans.
When the individual is later vaccinated, these non-protective antibodies are preferentially recalled, making the vaccine ineffective.
This study showed that antibody responses against SA cell-wall-associated antigens (CWAs) are non-opsonic, while antibodies against SA toxins are neutralizing.
Significantly, the protective characteristics of the antibody imprints accurately predict the failure of corresponding vaccines against CWAs and support vaccination against toxins.
In passive immunization platforms, natural anti-SA human antibodies reduce the efficacy of the human monoclonal antibodies suvratoxumab and tefibazumab, which is consistent with the results of their respective clinical trials.
Strikingly, in the absence of specific humoral memory responses, active immunizations are efficacious in both naive and SA-experienced mice.
Overall, this study points to a practical and predictive approach to evaluating and developing SA vaccines based on pre-existing humoral imprint characteristics.
“SA has been with humans a long time, so it’s learned how to be part-time symbiont, part-time deadly pathogen,” said senior author George Liu MD, PhD, professor in the Department of Pediatrics at UC San Diego School of Medicine, in a press release on January 16, 2024.
“If we’re going to develop effective vaccines against SA, we need to understand and overcome the strategies it uses to maintain this lifestyle.”
This study was funded, in part, by the U.S. National Institute of Health.
By U.S. regulatory provisions, the Centers for Disease Control and Prevention (CDC) announced today that the Advisory Committee on Immunization Practices (ACIP) meeting will be open to the public.
As of January 16, 2024, this agenda will include discussions on influenza, chikungunya (IXCHIQ®), COVID–19, meningococcal, pneumococcal, polio, HPV, RSV, combined Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus influenzae Type B Conjugate, and Hepatitis B vaccine (Vaxelis®), and vaccines to prevent diphtheria, tetanus, and pertussis.
Recommendation votes are scheduled for influenza vaccines, COVID–19 vaccines, and chikungunya vaccine.
A VFC vote is scheduled for vaccines to prevent diphtheria, tetanus, and pertussis.
The ACIP develops recommendations for U.S. immunizations.
The CDC Committee is mandated to establish and periodically review and, as appropriate, revise the list of vaccines for administration to vaccine-eligible children through the Vaccines for Children program, along with schedules regarding dosing interval, dosage, and contraindications to administration of vaccines.
These ACIP meeting agenda items are subject to change as priorities dictate. For more information on the meeting agenda, visit https://www.cdc.gov/vaccines/acip/meetings/index.html.
The ACIP digital meeting will be held on February 28, 2024, from 8 a.m. to 5 p.m. EST and on February 29, 2024, from 8 a.m. to 3 p.m. EST.
Interested persons or organizations are invited to participate by submitting written views, recommendations, and data. To accommodate the significant interest in participation in the oral public comment session of ACIP meetings, each speaker will be limited to three minutes, and each speaker may speak only once per meeting.

According to various health departments located on the East Coast of the United States, there have been several confirmed reports of measles infections in early 2024.
As of January 16, 2024, the Philadelphia Department of Public Health reported a measles cluster among eight unvaccinated residents.
Across the river from Philadelphia, the Camden County, New Jersey Health Department announced on January 13, 2023, that it was closely monitoring a measles case of a child who attended daycare.
On January 13, 2024, the Virginia Department of Health was notified of a confirmed case of measles in a person who traveled through Dulles and Reagan airports when returning from international travel.
Furthermore, the Delaware Division of Public Health reported on January 11, 2024, a potential measles exposure at the Nemours Children's Hospital in Wilmington. An investigation has identified up to 30 people exposed to the infectious individual in late December 2023.
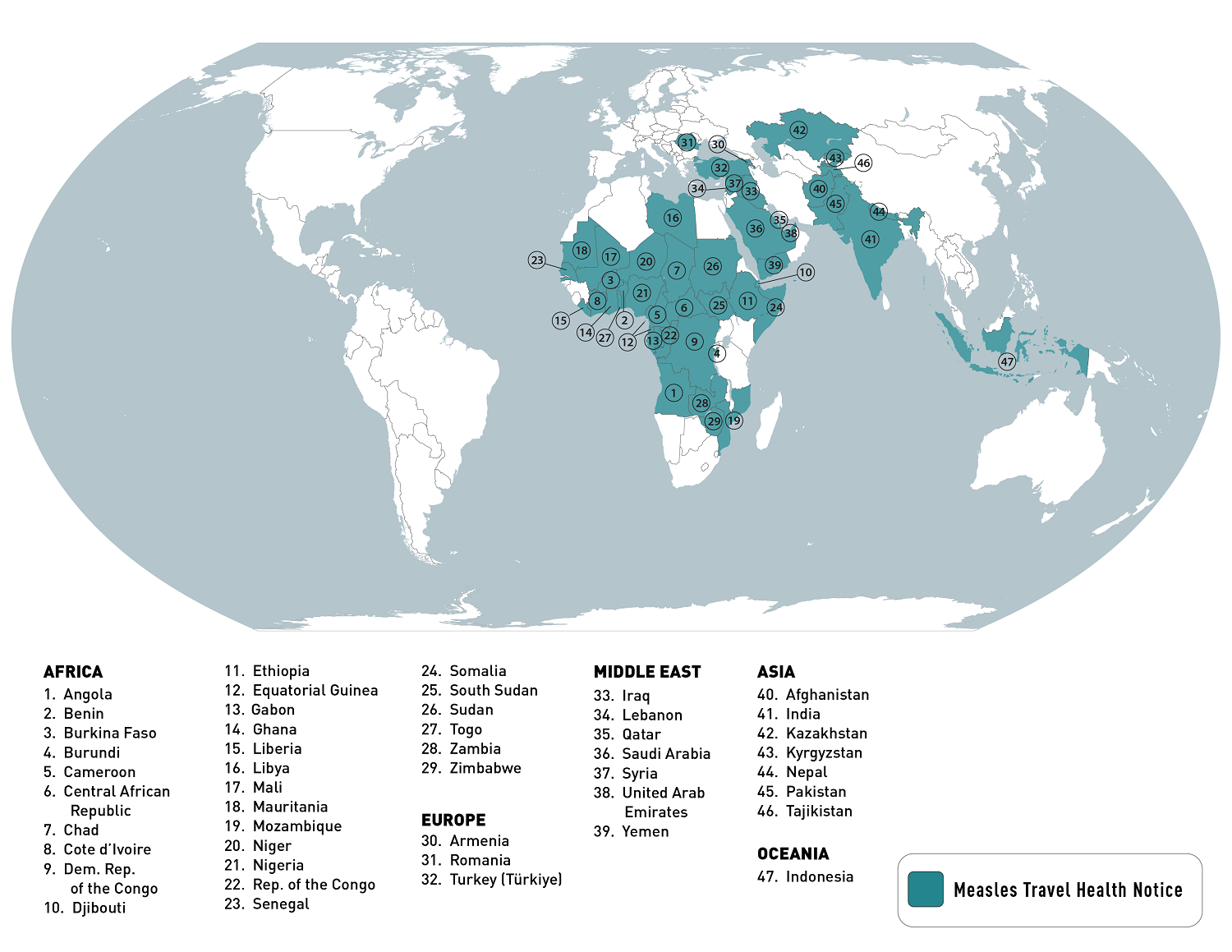
Most measles cases in the U.S. are related to international travel, says the U.S. Centers for Disease Control and Prevention (CDC). In 2023, there were 48 measles cases confirmed in twenty U.S. jurisdictions.
Last year, the CDC published a global Watch-Level 1, Practice Usual Precautions, Travel Health Notice in 2023, identifying measles outbreaks in 47 countries.
As of January 12, 2024, the CDC listed the top ten measles outbreaks led by Yemen and India over the past year.
Measles is a vaccine-preventable disease, says the CDC. A highly contagious virus causes it. The measles virus can live for up to two hours in an airspace after an infected person leaves an area. Moreover, people can spread measles up to four days before and four days after a rash.
Most clinics and pharmacies in the U.S. offer measles vaccination services.
A recent article published by the journal Science Direct highlights the importance of responses to public health emergencies, such as the increasing number of yellow fever (YF) cases in the Federative Republic of Brazil.
In a March 2024 article, researchers compared the detection and phylogenetic analysis of the YF virus in two neotropical primates (monkey), a Callithrix detected in the previous epidemic period (2016–2020) and a Callicebus nigrifons, which showed a new introduction of YF virus in Brazil during 2023.
In 2022, the Pan American Health Organization confirmed Brazil reported four deaths related to yellow fever infections.
Mosquitoes that transmit the yellow fever virus can be found throughout the Amazon and along most river basins in Brazil.
Evidence of an expanded range of yellow fever transmission in Brazil led the WHO and the U.S. CDC to broaden their vaccination coverage recommendations in 2017.
Since Brazil is a favorite destination for millions of international travelers, the U.S. CDC says travelers to Brazil should be updated on routine vaccines, including the YF vaccine.
YF vaccination is recommended for most travelers going to the states of Acre, Amapá, Amazonas, Distrito Federal (including the capital city, Brasília), Espírito Santo, Goiás, Maranhão, Mato Grosso, Mato Grosso do Sul, Minas Gerais, Pará, Paraná, Piauí, Rio de Janeiro (including the city of Rio de Janeiro and all coastal islands), Rio Grande do Sul,* Rondônia, Roraima, Santa Catarina, São Paulo (including the city of São Paulo and all coastal islands), Tocantins, and designated areas of Bahia.
The CDC's expanded YF vaccination recommendations for these states are preliminary. For updates, refer to the CDC Travelers' Health website.
"Even if your travel plans to Brazil are limited to popular urban areas like São Paulo or Rio de Janeiro, you are still at risk for yellow fever infection because the mosquito variety that carries the yellow fever virus has adapted to live in cities and bites during the day time," says Jeri Beales MSN RN, with Destination Health, located near Boston, MA.
"The good news is the yellow fever vaccine is very effective at preventing infections, and it protects for at least ten years, with some people only needing a single lifetime dose," added Beales.
Although Brazil does not require proof of vaccination against yellow fever for entry into the country, people planning to travel to other countries in South America (e.g., Colombia) could be required to show proof of yellow fever vaccination before exiting Brazil.
As of January 16, 2024, several yellow fever vaccines have been authorized by various countries. In the U.S., the YF-VAX® vaccine is available at travel clinics.

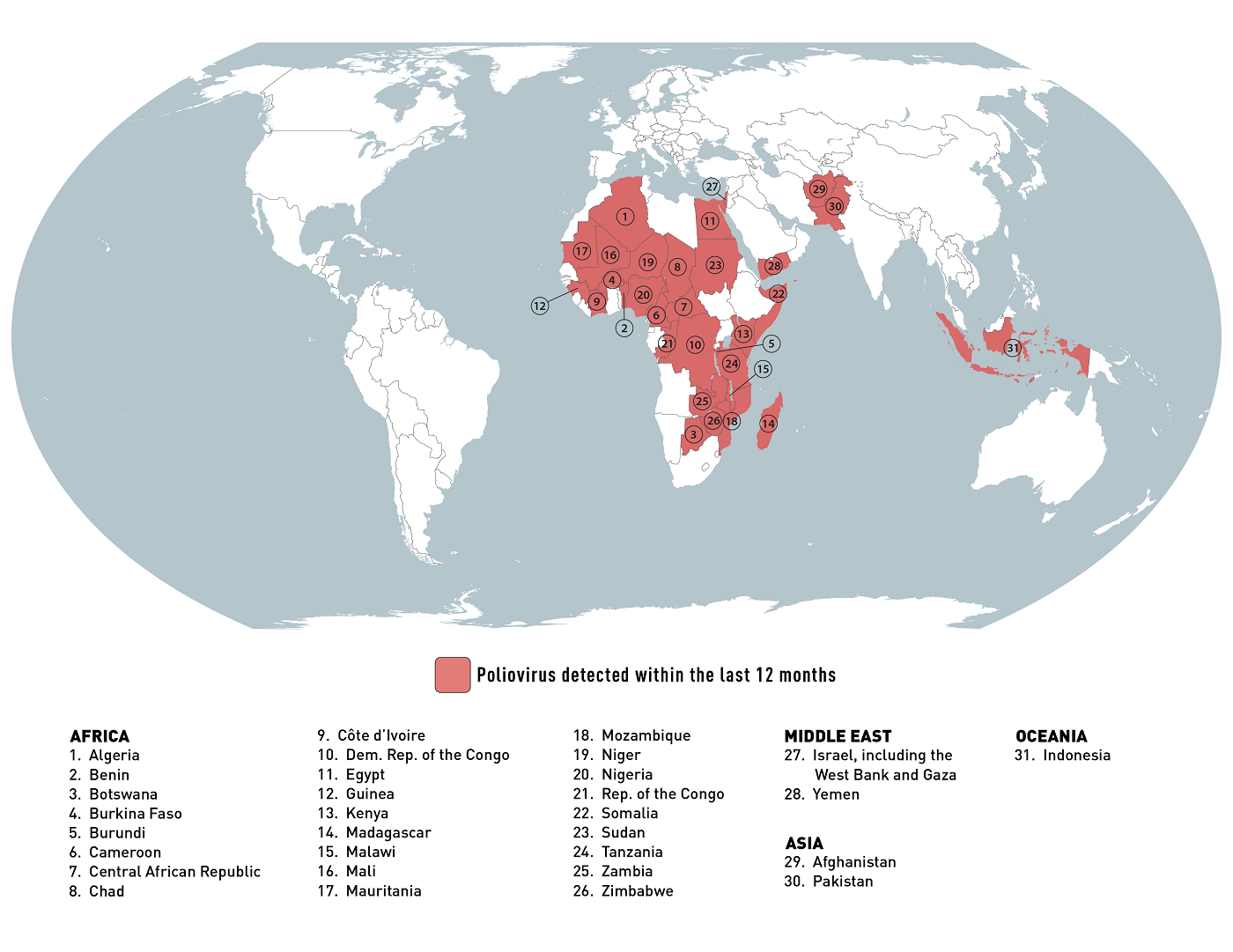
The U.S. Centers for Disease Control and Prevention (CDC) recently reissued its Level 2 - Practice Enhanced Precautions Global Polio notice.
As of January 5, 2024, the CDC's Travel Health Notice confirmed that polio and poliovirus are health risks in about thirty-one countries that have recently reported outbreaks.
The CDC says that adults who previously completed the whole, routine polio vaccine series may receive a single, lifetime booster dose of the polio vaccine before traveling to any of these destinations.
Any other traveler should ensure they are up-to-date on their polio vaccination schedule.
In the U.S., the IPV vaccine has been deployed since 2000.
Jeri Beales, MSN RN at Destination Health Travel Clinic in Natick, MA, 01760, told Precision Vaccinations, "It's a common misconception that polio has been eradicated worldwide. We forget that polio is still out there because cases are so rare in the U.S. Decades of vaccination efforts in the U.S. have kept infections at bay; still, sadly, polio has regained a foothold in various countries."
"So, if you have plans to travel to a country experiencing a polio outbreak, you may be putting yourself and your family at risk. Adults need to receive a one-time additional dose of polio before traveling to any high-risk countries because the vaccines you received as a child may no longer be protective. Like a lot of vaccines, the effectiveness wears off with time."
"And don't forget, if you're traveling with children, check with their pediatrician to ensure they are up-to-date on their polio immunizations.
In late 2023, the WHO announced that the risk of the international spread of poliovirus remains a Public Health Emergency of International Concern and recommended its extension and Temporary Recommendations for three months.
According to the CDC, most people with polio do not feel sick. Some people have only minor symptoms, such as fever, tiredness, nausea, headache, nasal congestion, sore throat, cough, neck and back stiffness, and arm and leg pain.
However, in rare cases, polio infection causes permanent loss of muscle function. Polio can be fatal if the muscles used for breathing are paralyzed or if there is an infection of the brain.
Note: This news article was updated with the provider's perspective on Jan.16, 2024.
The Annals of Internal Medicine recently confirmed the Advisory Committee on Immunization Practices (ACIP) voted to approve the Recommended Adult Immunization Schedule for Ages 19 Years or Older.
The 2024 adult immunization schedule summarizes the U.S. Centers for Disease Control and Prevention (CDC) ACIP recommendations in the cover page, tables, notes, appendix, and addendum.
The complete ACIP recommendations for each vaccine are available at this CDC link.
As of January 12, 2024, the new schedule has also been approved by the director of the CDC and by the American College of Physicians, the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American College of Nurse-Midwives, the American Academy of Physician Associates, the American Pharmacists Association, and the Society for Healthcare Epidemiology of America.