Search API
Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) today announced it is awarding $1.7 million to Syntiron to develop a maternal vaccine that targets Escherichia coli and Klebsiella pneumoniae.
Syntiron's Alloy-EK vaccine leverages iron receptor proteins (IRPs) as vaccine targets. IRPs are highly genetically conserved, which means they are less likely to change since they perform essential functions.
This makes these proteins reliable vaccine targets.
Syntiron developed the Alloy Platform to manufacture and formulate IRP vaccines safely that maintain robust immunity and cover a broad range of bacterial strains.
Neonatal sepsis is a life-threatening response to bloodstream infections that occur in newborns fewer than 28 days old. Due to their immature immune systems, newborns are particularly susceptible to infections.
Since neonatal sepsis progresses rapidly, the risk of death from neonatal sepsis increases by 7.6% every hour a treatment is delayed.
The BARNARDS study estimated that 2.5 million neonates or infants die in the first month of life of sepsis annually, with the most significant burden in low- and middle-income countries.
"The bacteria targeted by this vaccine are a tremendous burden on public health for babies, children, and adults worldwide," said Lisa Herron-Olson, Ph.D., Managing Director of Syntiron, in a press release on January 18, 2024.
"Pregnant women are at particularly high risk of infection by these same bacteria that can cause neonatal sepsis in newborn babies."
"Preventing these infections by vaccination offers substantial potential health benefits to mothers and babies while reducing the spread of antimicrobial resistance."
Syntiron's innovation with the Alloy Platform leveraged molecular, bioinformatic, and immunological principles to engineer simpler proteins enriched in linear, immunodominant, and highly conserved peptides derived from IRPs.
Founded in 2004, Syntiron is a biotechnology company based in Saint Paul, MN, and has several vaccines in development that target bacterial infections.=

A non-peer-reviewed study supported by the U.S. Food and Drug Administration (FDA) as part of the SafeRx Project, a joint initiative of the Centers for Medicare & Medicaid Services, published on January 17, 2024, did not observe clear, consistent evidence of increased stroke risk following high to dose or adjuvanted influenza vaccination across the three flu seasons 2016 to 2019.
The statistically significant associations identified in this study were not consistently observed across outcomes, risk windows, age subgroups, or seasons.
Furthermore, the clinical significance of any potential risk of stroke following influenza vaccination must be carefully considered, together with the significant benefits of receiving an annual flu shot.
While studies have shown that influenza infections increase the risk of stroke, this study only included vaccinated cases and did not account for the protective effect of vaccination against infections. Address correspondence to Yun Lu, MS, PhD, Silver Spring, MD 20993; email: [email protected].

Global spending and demand for medicines will increase over the next five years to approximately $2.3 trillion by 2028 as more patients get access to new and better medicines, according to the IQVIA Institute.
On January 17, 2024, IQVIA published a new report for Human Data Science titled “The Global Use of Medicines 2024 – Outlook through 2028.”
This updated projection raises the growth outlook by 2% despite lower expectations for COVID-19 vaccines and therapeutics.
“The continued growth in spending is driven by an increase in the volume of medicines (vaccines), which reflects that more patients globally are getting access to novel medicines with better clinical outcomes,” said Murray Aitken, SVP and executive director of the IQVIA Institute for Human Data Science, in a press release.
Highlights of this report include, but are not limited to, the following:
Global use of medicines grew by 14% over the past five years, and a further 12% increase is expected through 2028, bringing annual use to 3.8 trillion defined daily doses.
This increase in growth outlook is driven by more patients getting treated with better medicines, especially in immunology, endocrinology, and oncology.
Medicine use in Latin America and Asia will grow faster than in other regions over the next five years.
Using estimated net prices, the updated outlook for the U.S. market is being raised to 2-5% CAGR through 2028, reflecting higher recent growth and expected further increased patient use of higher-value therapies.
IQVIA (NYSE: IQV) is a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry.
The Federative Republic of Brazil's Ministry of Health recently confirmed it is the first country in the world to offer Takeda's QDENGA® dengue vaccine in a universal public system.
QDENGA (TAK-003) is a tetravalent vaccine [Live, Attenuated] that does not require pre-admision testing.
As of January 15, 2024, the Ministry of Health forecasts that 5.2 million doses will be delivered between February and November 2024.
The current expectation is that around 3.2 million people will be vaccinated in 2024.
In 2023, the WHO's Strategic Advisory Group of Experts on Immunization confirmed QDENGA had demonstrated efficacy against all four serotypes of the dengue virus in baseline seropositive children (4-16 years) in endemic countries and against serotypes 1 and 2 in baseline seronegative children.
As of January 17, 2024, QDENGA is authorized in several countries but not the United States.

The Philadelphia Health Department reported another new case of measles, the ninth confirmed measles case in 2024.
Today’s (Jan. 16, 2024) case is the 5th measles case associated with the previously reported daycare outbreak.
According to the U.S. Centers for Disease Control and Prevention (CDC), most measles outbreaks in the United States are related to unvaccinated international travelers.
Since measles is a vaccine-preventable disease, the Health Department is coordinating several vaccination opportunities to ensure that children and adults who need the measles, mumps, and rubella (MMR) vaccine can be vaccinated for free in their community.
People seeking MMR vaccination do not need any identification; a piece of mail with an address on it will qualify them as a Philadelphia resident, and insurance is not required to be vaccinated.
In addition to the measles outbreak in Philadelphia, cases have recently been reported in New Jersey, Delaware, and Virginia.
On the West Coast, six measles cases were recently confirmed in Clark and Wahkiakum counties in Washington in January 2024.
Globally, the CDC identified 47 countries with recent measles outbreaks led by Yemen and India.
In the U.S., measles vaccination services are generally offered at clinics and pharmacies.

Biological E. Limited today announced that the World Health Organisation (WHO) has granted an Emergency Use Listing (EUL) to their CORBEVAX® vaccine, India's first indigenously developed COVID-19 vaccine.
As of January 16, 2024, there are 13 COVID-19 vaccines granted EUL by the WHO.
CORBEVAX's antigen's initial construct and production process were developed at Texas Children's Hospital in Houston, Texas, led by Drs. Bottazzi and Hotez and in-licensed from BCM Ventures.
CORBEVAX is a protein sub-unit vaccine not produced with mRNA technology.
The Drugs Controller General of India (DCGI) has already approved CORBEVAX for restricted use in emergencies among adults, adolescents, and young children in a sequential manner from December '21 to April '22, as well as India's first heterologous COVID-19 booster shot for adults age 18 and above in June '22.
BE supplied 100 Million Doses of CORBEVAX to the Government of India.
Ms. Mahima Datla, Managing Director of Biological E. Limited, said in a press release, "We understand that several countries come under a lot of fiscal pressure when dealing with COVID-19. We aim to reach the people in those countries with CORBEVAX, just as we have done with all our other vaccines."
"Our commitment is to provide affordable and accessible high-quality vaccines, and the WHO EUL lays a path for us to make that possible."
Ms. Mahima further added, "While several companies which entered the field of vaccine development & manufacturing during the COVID-19 pandemic exited soon afterward either due to paucity of funds or lack of success, BE continues to remain committed to develop and provide access to high-quality, affordable vaccines globally by constantly enlarging its portfolio of offerings."
As of January 17, 2024, CORBEVAX is not U.S. FDA-approved or available in the U.S.

The Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) today announced it is awarding $633k to Intravacc to support the development of the Avacc® 11 vaccine candidate that prevents Neisseria gonorrhoeae bacterial infections.
This CARB-X award supports the development of Intravacc's meningococcal outer membrane vesicle (OMV) vaccine that carries several important gonococcal antigens to prevent Neisseria gonorrhoeae (gonococci) infections.
Through the tailored gonococcal antigens on the surface of the meningococcal OMV, Intravacc anticipates a significant enhancement in the vaccine candidate's efficacy against gonorrhea.
"Drug-resistant strains of Neisseria gonorrhoeae have evaded all but one existing antibiotic (ceftriaxone)," said Erin Duffy, PhD, R&D Chief of CARB-X, in a press release on January 16, 2024.
"Vaccines are powerful tools in the prevention of bacterial infections."
"With an appropriate vaccination strategy, Intravacc's vaccine project, if successful, could prevent the disease and significantly curb the spread of resistant bacteria across the globe."
The WHO Global Health Sector Strategy on Sexually Transmitted Infections (STI) has set goals for reducing gonorrhea incidence by vaccination by 90% by 2030. Gonorrhea is the world's second most reported STI, impacting about 82 million adults annually.
As of 2024, the U.S. FDA has not approved a gonorrhea vaccine.
However, a Research Letter published by the JAMA Network Infectious Diseases on August 31, 2023, concluded that the Outer Membrane Vesicles-based meningococcal group B vaccine was 47% (95% CI, 13%-68%) effective in preventing gonorrhea in young adults.
On November 10, 2023, the U.K.'s Joint Committee on Vaccination and Immunisation agreed that a targeted vaccination program should be initiated using the 4CMenB (Bexsero®) vaccine for the prevention of gonorrhea in those who are at most significant risk of infection.
Intravacc is a global contract development and manufacturing organization at the Utrecht Science Park Bilthoven in the Netherlands. Intravacc is developing vaccines that target a range of diseases.

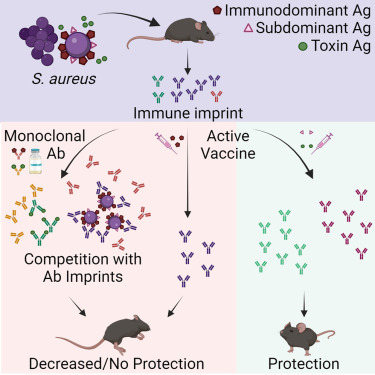
In a new study published in Cell Reports Medicine, researchers at the University of California San Diego School of Medicine explained why people would benefit from a saphylococcus aureus (SA) vaccine.
While SA causes many dangerous health complications, including wound and bloodstream infections, the bacterium is also a normal part of the healthy human microbiome, where it lives peacefully in the nose and on the skin.
These researchers tested a new hypothesis that SA bacteria can trick the body into releasing non-protective antibodies when they first colonize or infect humans.
When the individual is later vaccinated, these non-protective antibodies are preferentially recalled, making the vaccine ineffective.
This study showed that antibody responses against SA cell-wall-associated antigens (CWAs) are non-opsonic, while antibodies against SA toxins are neutralizing.
Significantly, the protective characteristics of the antibody imprints accurately predict the failure of corresponding vaccines against CWAs and support vaccination against toxins.
In passive immunization platforms, natural anti-SA human antibodies reduce the efficacy of the human monoclonal antibodies suvratoxumab and tefibazumab, which is consistent with the results of their respective clinical trials.
Strikingly, in the absence of specific humoral memory responses, active immunizations are efficacious in both naive and SA-experienced mice.
Overall, this study points to a practical and predictive approach to evaluating and developing SA vaccines based on pre-existing humoral imprint characteristics.
“SA has been with humans a long time, so it’s learned how to be part-time symbiont, part-time deadly pathogen,” said senior author George Liu MD, PhD, professor in the Department of Pediatrics at UC San Diego School of Medicine, in a press release on January 16, 2024.
“If we’re going to develop effective vaccines against SA, we need to understand and overcome the strategies it uses to maintain this lifestyle.”
This study was funded, in part, by the U.S. National Institute of Health.
By U.S. regulatory provisions, the Centers for Disease Control and Prevention (CDC) announced today that the Advisory Committee on Immunization Practices (ACIP) meeting will be open to the public.
As of January 16, 2024, this agenda will include discussions on influenza, chikungunya (IXCHIQ®), COVID–19, meningococcal, pneumococcal, polio, HPV, RSV, combined Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus influenzae Type B Conjugate, and Hepatitis B vaccine (Vaxelis®), and vaccines to prevent diphtheria, tetanus, and pertussis.
Recommendation votes are scheduled for influenza vaccines, COVID–19 vaccines, and chikungunya vaccine.
A VFC vote is scheduled for vaccines to prevent diphtheria, tetanus, and pertussis.
The ACIP develops recommendations for U.S. immunizations.
The CDC Committee is mandated to establish and periodically review and, as appropriate, revise the list of vaccines for administration to vaccine-eligible children through the Vaccines for Children program, along with schedules regarding dosing interval, dosage, and contraindications to administration of vaccines.
These ACIP meeting agenda items are subject to change as priorities dictate. For more information on the meeting agenda, visit https://www.cdc.gov/vaccines/acip/meetings/index.html.
The ACIP digital meeting will be held on February 28, 2024, from 8 a.m. to 5 p.m. EST and on February 29, 2024, from 8 a.m. to 3 p.m. EST.
Interested persons or organizations are invited to participate by submitting written views, recommendations, and data. To accommodate the significant interest in participation in the oral public comment session of ACIP meetings, each speaker will be limited to three minutes, and each speaker may speak only once per meeting.