Search API
According to a Clinician Outreach and Communication Activity COCA Now message published today, the U.S. Centers for Disease Control and Prevention (CDC) was notified of 23 measles cases between December 2023 and January 23, 2024.
On January 24, 2024, this COCA Now email stated these cases include seven direct importations of measles by international travelers and two outbreaks with more than five cases each.
Specifically, most of the recent measles cases were among children and adolescents who had not received a measles-containing vaccine, even if they were age-eligible.
The increased number of measles importations in the U.S. reflects a rise in global measles outbreaks.
Over the past year, the CDC says Yemen (23,066) and India (13,997) have reported the most measles cases.
In the U.S., the Philadelphia Department of Public Health recently reported a measles cluster among unvaccinated residents. Nine measles cases have been confirmed in Philadelphia locations in 2024.
The Georgia Department of Public Health confirmed a measles case in an unvaccinated Atlanta resident.
The Virginia Department of Health was notified of a confirmed case of measles in a person who traveled through Northern Virginia airports.
In greater Kansas City, Missouri, a resident at the Kansas City International Airport and North Kansas City Hospital was infected with the measles virus.
And in Washington, six measles cases were confirmed in January 2024.
In the U.S., various measles vaccines, such as GSK's Priorix, are generally available at local pharmacies.
COCA Now emails will be sent as soon as possible after the CDC publishes new content, ensuring clinicians are updated.
Novavax, Inc. today announced that the United Kingdom's (U.K.) Medicines and Healthcare products Regulatory Agency (MHRA) granted marketing authorization for Nuvaxovid XBB.1.5 dispersion for injection, COVID-19 Vaccine (recombinant, adjuvanted) for active immunization to prevent COVID-19 in individuals aged 12 and older.
Recent data indicate Novavax's vaccine can stimulate both arms of the immune system and induce a broad response against circulating variants.
"Today's MHRA authorization is recognition of the role our vaccine can have in protecting the British public against COVID-19 this year," said John C. Jacobs, President and Chief Executive Officer, Novavax, in a press release on January 24, 2024.
"We are in ongoing conversations with additional U.K. partners to identify potential opportunities to offer our protein-based non-mRNA COVID-19 vaccine to all eligible individuals who want one."
"We believe this is critical to supporting long-term, broad uptake of a seasonal COVID-19 vaccine in the U.K."
In clinical trials, the most common adverse reactions associated with Novavax's prototype COVID-19 vaccine (NVX-CoV2373) included headache, nausea or vomiting, muscle pain, joint pain, injection site tenderness, injection site pain, fatigue, and malaise.
If people are concerned about an adverse event, it should be reported on a Yellow Card. Reporting forms and information can be found at https://coronavirus-yellowcard.mhra.gov.uk/.
The U.K. authorization was based on non-clinical data showing that Novavax's updated COVID-19 vaccine induced functional immune responses for XBB.1.5, XBB.1.16, and XBB.2.3 variants.
Additional non-clinical data demonstrated that Novavax's vaccine-induced neutralizing antibody responses to subvariants JN.1, BA.2.86, EG.5.1, FL.1.5.1, and XBB.1.16.6, as well as CD4+ polyfunctional cellular (T-cell) responses against EG.5.1 and XBB.1.16.6.
In 2023, the U.S. Food and Drug Administration amended its authorization for Novavax COVID-19 Vaccine, Adjuvanted for use in individuals 12 and older, to include the 2023-2024 formula.
Novavax COVID-19 vaccine brands include Nuvaxovid, NVX-CoV2601, CovoVax, NVX-CoV2373, and TAK-019, Trademark filing #90813423.

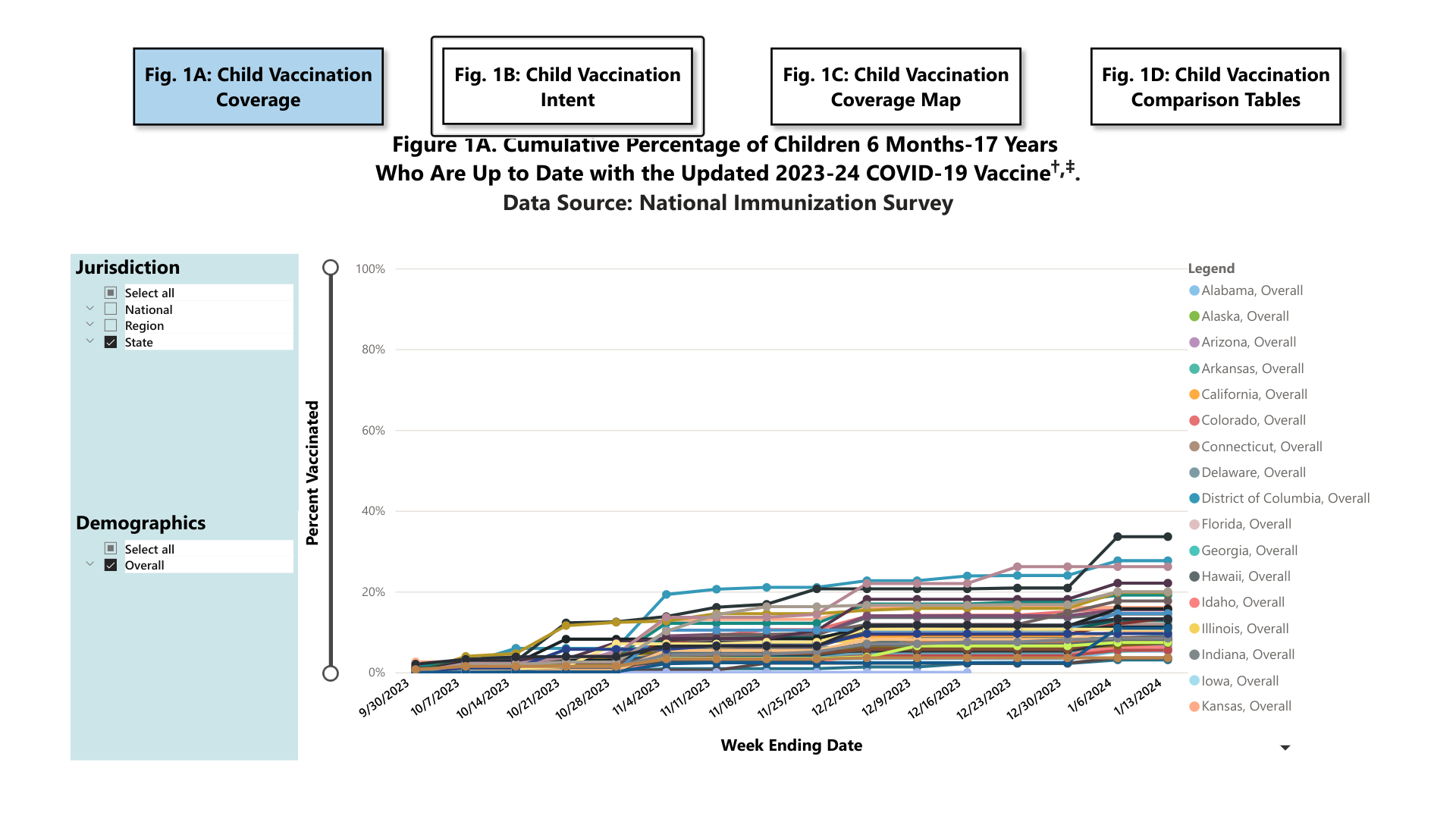
According to the U.S. Centers for Disease Control and Prevention (CDC) COVIDVaxView data, about 11% (CI 10.1 to 11.8) of children under the age of 18 are up to date with their COVID-19 vaccinations.
From a state perspective, as of January 23, 2024, Massachusetts has reached about a third of children with COVID-19 vaccinations.
Furthermore, Louisana was the lowest-ranked state, with only 3.1% (CI 1.2 to 5.0) of children up to date.
The CDC says up to date with the updated 2023-24 COVID-19 vaccine is defined as receipt of at least one vaccination since September 14, 2023, for children ≥5 years; for children <5 years, up-to-date status was defined based on the current recommendations that also take into account number of doses and brand of vaccine.
Up-to-date status was determined by survey questions on the month and year of the most recent COVID vaccine, and for children <5 years, the total number of COVID vaccinations received and brand of the most recent COVID vaccine.
Each week, estimates for prior weeks are recalculated by the CDC using the additional interviews conducted that week.
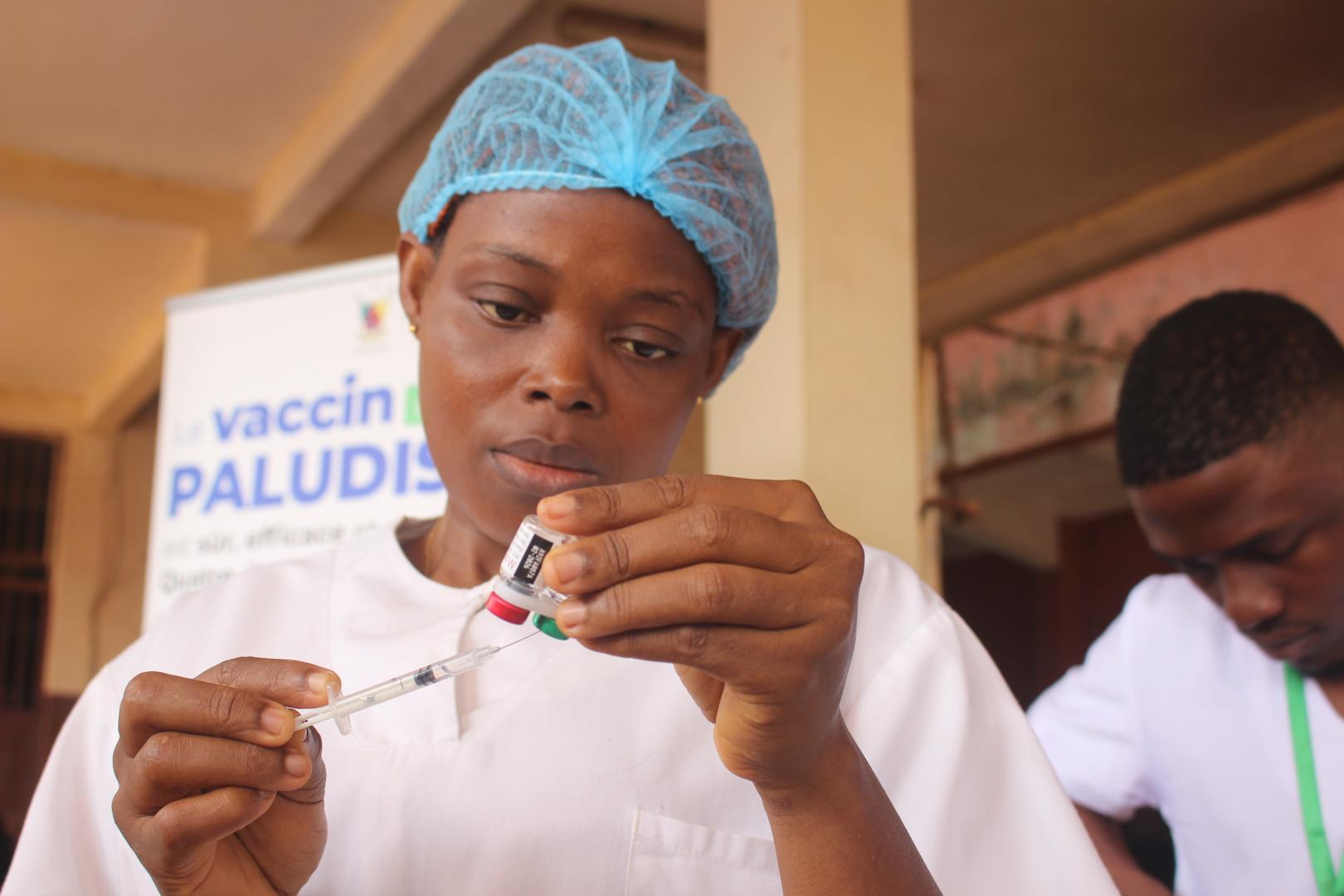
The Ministry of Health of the Republic of Liberia today announced the arrival of 112,000 doses of the Mosquirix™ (RTS,S) malaria vaccine.
GSK's Mosquirix vaccine has been piloted in Africa since 2019 and has been shown to be safe and effective, reducing severe malaria by 30% and malaria deaths by 13%.
With two malaria vaccines now approved for use in Africa, Liberia's fight against this preventable mosquito-borne disease may save thousands of children's lives. Malaria vaccinations are scheduled to launch in April 2024.
"The arrival of this life-saving vaccine is a game changer in our fight against malaria," said Adolphus Clarke, Director of the Expanded Programme on Immunization at the Ministry of Health of Liberia, in a press release on January 23, 2024.
"We are committed to ensuring that every child in Liberia has access to this vaccine and is protected from this deadly disease."
According to the World Health Organization, eleven countries saw an estimated 249 million malaria cases and 426,000 deaths in 2022.
In the United States, about ten locally acquired malaria cases were reported in 2023.
However, malaria vaccines are not available in the U.S. as of January 2024.

The Coalition for Epidemic Preparedness Innovations (CEPI) today announced Serum Institute of India Pvt. Ltd (SII) has joined CEPI's network of vaccine producers in the Global South to support more rapid, agile, and equitable responses to future public health disease outbreaks.
To prepare for such a scenario, CEPI is investing up to $30 million to build upon SII's proven track record of rapid response to infectious disease outbreaks, expanding the company's ability to swiftly supply investigational vaccines in the face of epidemic and pandemic threats.
Created by CEPI to expand the global footprint of vaccine production, the manufacturing network focuses on vaccine makers in the Global South near areas at high risk of outbreaks caused by deadly viral threats like Lassa Fever, Nipah, Disease X, and other pathogens with epidemic or pandemic potential prioritized by CEPI.
Dr. Richard Hatchett, CEO of CEPI, said in a press release on January 23, 2024, "As part of CEPI's global manufacturing network, SII's world-renowned manufacturing and rapid response capabilities are poised to play a critical role in enabling swift and equitable access to affordable outbreak vaccines for the Global South."
Given SII's already proven production capabilities, the company may be called upon to promptly supply investigational vaccines for preclinical and clinical testing and large-scale supply in the event of an outbreak.
Shortening the time taken to manufacture and validate the first batches of experimental vaccines will be vital to enabling a response to an escalating outbreak within just 100 days – a goal created by CEPI and embraced by the G7, G20, and industry leaders – and could help stop a future pandemic in its tracks.

The WHO European Region confirmed today the measles virus spread in 41 of its 53 Member States in 2023. Among the countries most affected in the Region, the Republic of Kazakhstan has recorded the highest incidence, with 13,677 measles cases in 2023.
The majority of Kazakhstan measles cases were in children, 11,300.
As of January 23, 2024, there are 2,167 children in a Kazakhstan hospital with measles, 27 of them in a serious condition.
To implement measles vaccination campaigns, the Kazakhstan government purchased an additional 1.5 million doses of MMR vaccine in 2023.
"We have seen in the Region not only a 30-fold increase in measles cases ....This is concerning," explained Dr. Hans Henri P. Kluge, WHO Regional Director for Europe, in a press release in 2023.
"Vaccination is the only way to protect children from this potentially dangerous disease."
"Urgent vaccination efforts are needed to halt transmission and prevent further spread."
"It is vital that all countries are prepared to rapidly detect and respond promptly to measles outbreaks, which could endanger progress towards measles elimination," added Dr. Kluge.
In the United States, several cities, such as Atlanta, Kansas City, Philadelphia, and Wilmington, have reported measles cases in 2024.
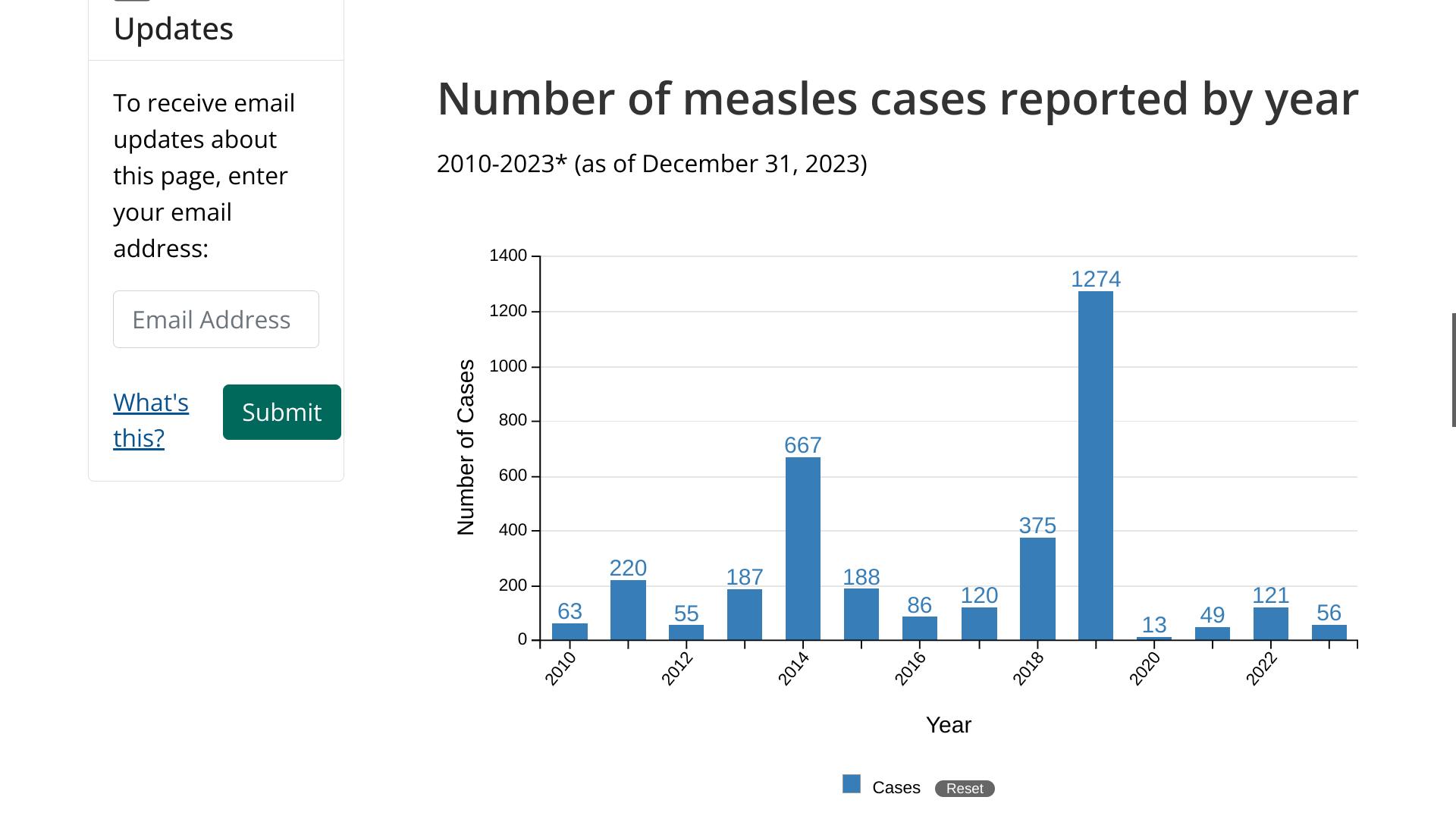
The U.S. CDC says most measles cases in the U.S. are travel-related. In 2023, there were 56 measles cases reported by 20 U.S. jurisdictions.
Measles is a vaccine-preventable disease. Various measles vaccines are available at local pharmacies in the U.S.

HDT Bio Corp. today announced that it has partnered with the Biomedical Advanced Research and Development Authority (BARDA), under Project NextGen's Enabler's program.
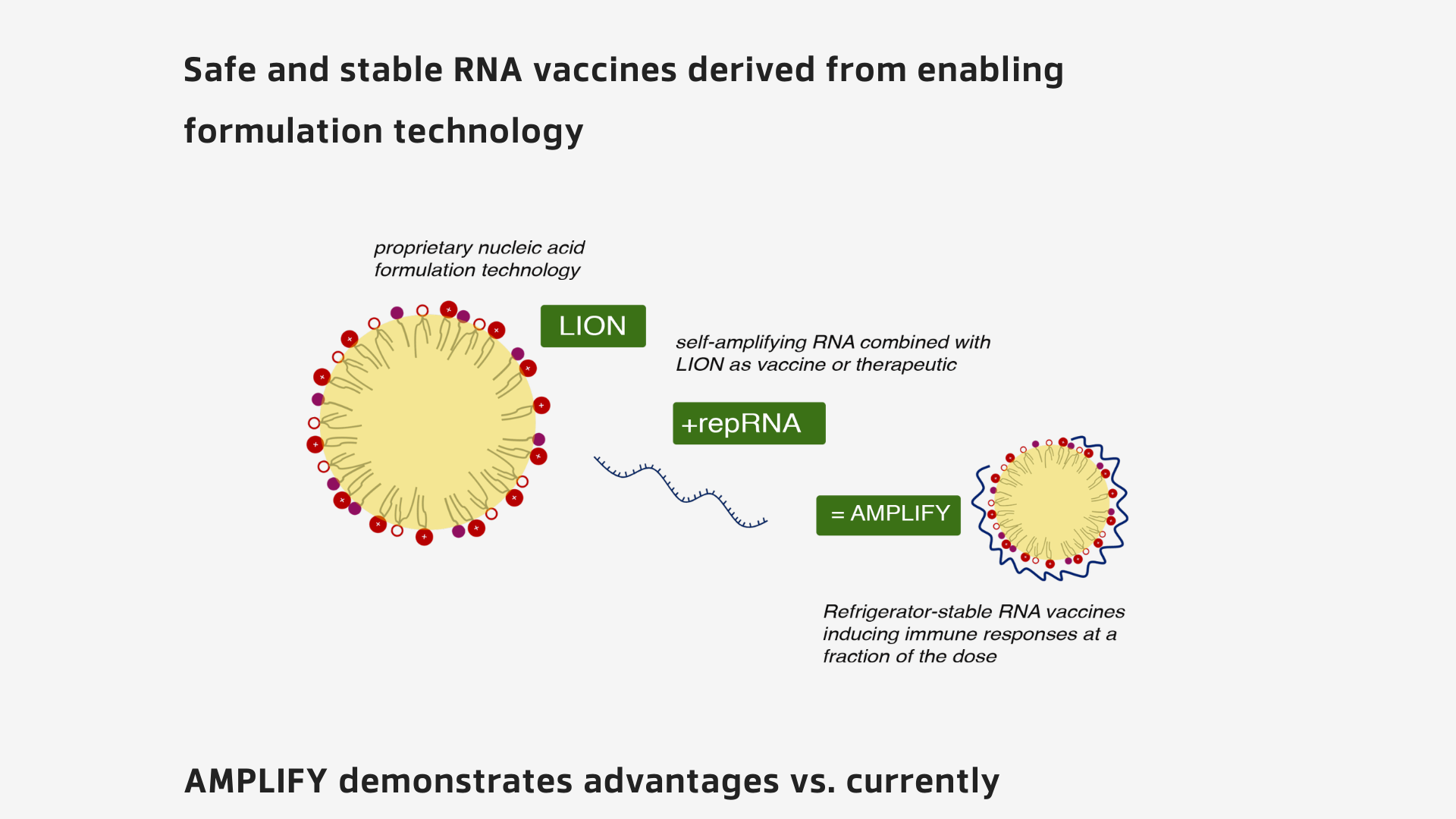
Through this BARDA partnership, HDT Bio has received a $749,000 contract, supporting proof-of-concept studies for on-demand manufacturing and release processes that use HDT Bio's LION™ formulation for RNA vaccine production.
These studies will provide proof-of-concept data on the LION platform in support of on-demand manufacturing and release processes that enable rapid production of 1000 repRNA vaccine doses in seven days.
LION is a proprietary nanoemulsion tailored for RNA-based vaccines and therapeutics production designed to enable facile formulation and faster manufacturing compared to the characteristics of standard lipid nanoparticles used in existing vaccine products.
"We look forward to contributing to the broader access to treatment and prevention strategies of future infectious diseases," commented Steven Reed, Ph.D., Co-founder and Chief Executive Officer of HDT Bio, in a press release on January 23, 204.
With an initial investment of $5 billion, Project NextGen intends to accelerate and streamline the development of the next generation of vaccines and treatments through public-private collaborations.
The Project NextGen Enabler's program aims to advance next-generation vaccine and therapeutics technologies, including the development of innovative cGMP manufacturing of vaccines that decrease costs, speed production, increase efficacy, and improve access.
Previous BARDA - Project NextGen awards include, but are not limited to, $8.5 million to CastleVax for a vector-based intranasal vaccine candidate, $10 million to Gritstone Bio for a self-amplifying mRNA vaccine candidate, and $9.27 million to Vaxart's evaluation of its oral pill XBB COVID-19 vaccine candidate.
Project NextGen is separated from the Disease X initiative.
The Republic of Cameroon today launched the Mosquirix™ (RTS,S) malaria vaccine into its routine national immunization services, becoming the first country to do so outside the malaria vaccine pilot program previously carried out in the African countries of Ghana, Kenya, and Malawi.
The launch on January 22, 2024, came after Cameroon received 331,200 Mosquirix doses in November 2023. Additional malaria vaccines are expected in 2024.
“The vaccine is an additional tool for malaria control. The country has chosen it (Mosquirix) based on its pre-qualification, ensuring guaranteed quality, efficacy, and safety for inclusion in the vaccination program,” said Dr. Shalom Ndoula, Permanent Secretary of the Expanded Programme on Immunization in Cameroon, in a press release.
“It will specifically target all children aged six months as of December 2023.”
In 2023, the Pan American Health Organization estimated that approximately 41 million people in twenty-one Latin American countries are at risk for malaria.
In the U.S., the Centers for Disease Control and Prevention confirmed about ten locally-acquired malaria cases in 2023.
Two approved malaria vaccines are available in certain countries in 2024 but not in the United States.