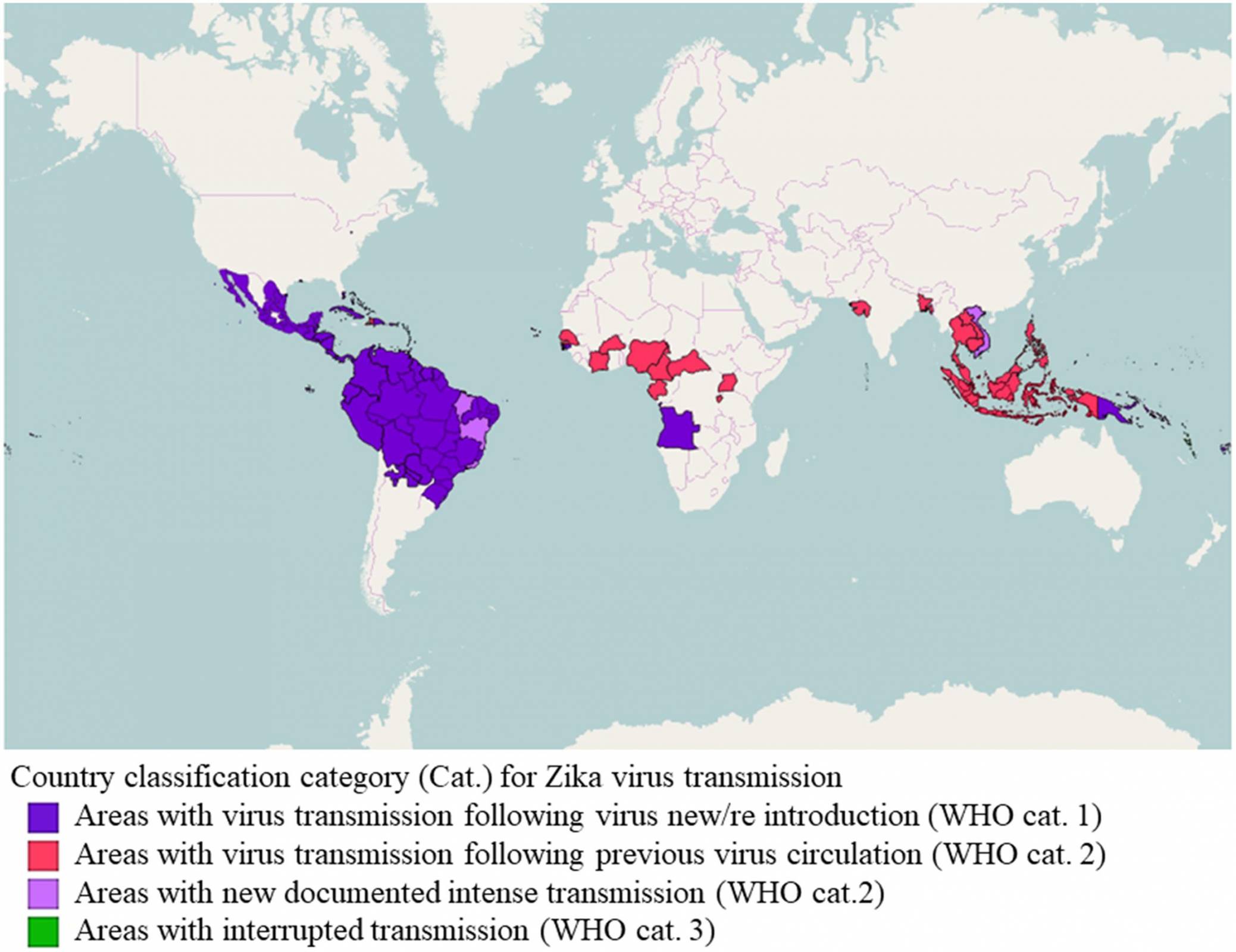
Precision Vaccinations Business
Ownership
Precision Vax, LLC is a 100% woman-owned private enterprise founded in 2016. Karen McClorey Hackett, Precision Vax's president, is committed to transparency in our ownership structure and revenue sources. Karen launched her career with IBM and has invested in digital health enterprises since 1997. Here is Karen's LinkedIn profile.
Revenues
PVax's primary revenues come from advertisers. Our editorial team has no relationship or communications with any advertiser. Our dominant digital advertiser is Google Adsense. Please note that advertising networks such as Adsense integrate user-tracking capabilities that may not be obvious to users. Google Ads Terms & Conditions are published at this link. PVax also integrates lead-generation business agreements for clinical trials, diagnostic tests, and vaccine appointments.
Brands
InstantRx™ trademark is owned by Precision Vax LLC.
Note: PVax was a customer of GoodRx Inc. until 2019. On Feb. 1, 2023 - FTC Enforcement Action to Bar GoodRx from Sharing Consumers' Sensitive Health Info for Advertising. Under the proposed FTC order, GoodRx will pay a $1.5 million civil penalty for failing to report its unauthorized disclosure of consumer health data to Facebook, Google, and other companies. GoodRx Inc. published a response.