Search API
The Maryland Department of Health and Montgomery County health officials recently announced a positive case of measles in a resident who had traveled through Washington Dulles International Airport International Terminal on January 27, 2024.
One case of measles was identified in Maryland in 2023 and five in 2019.
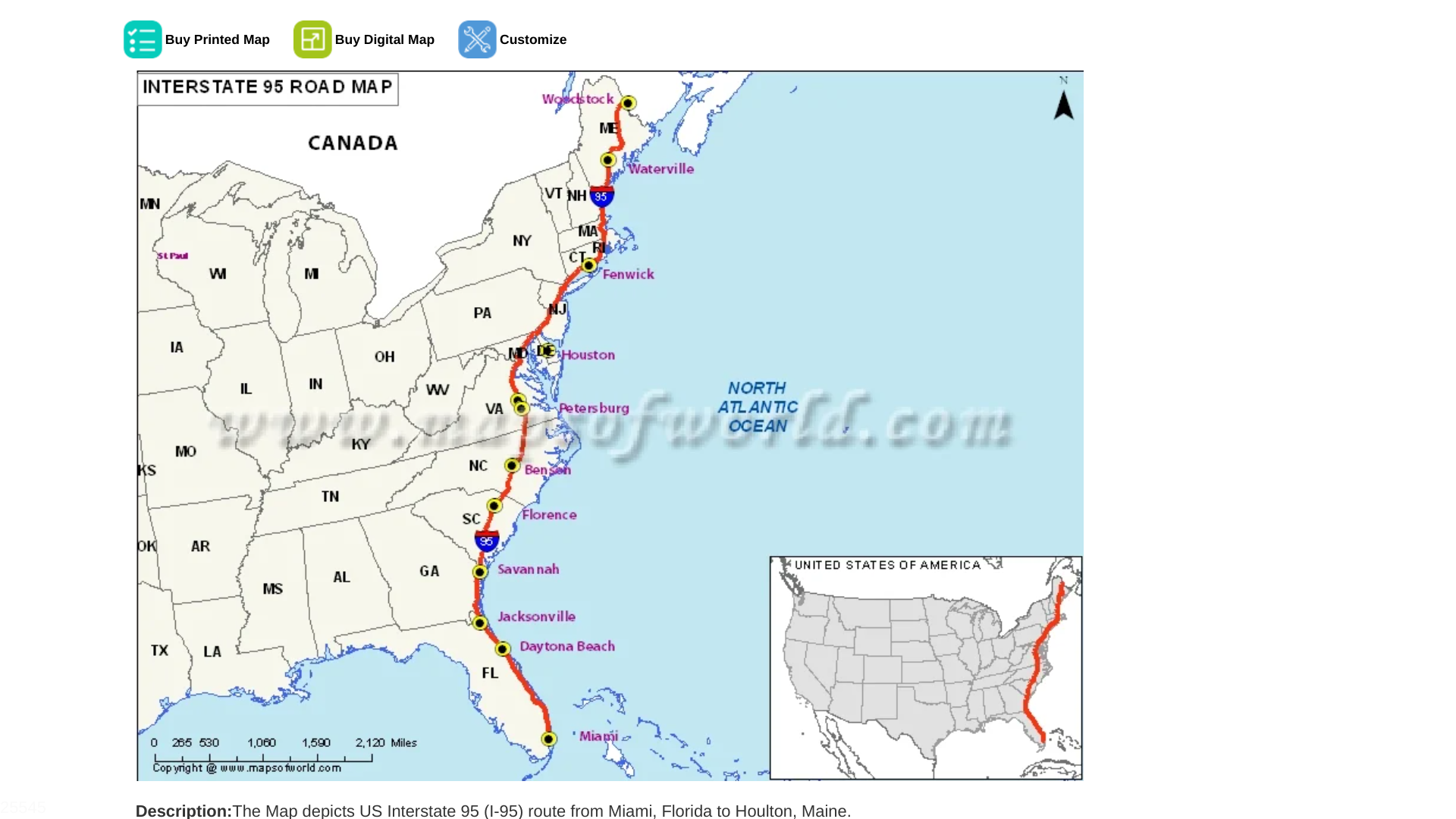
As of February 3, 2024, numerous measles cases have been reported along the I-95 highway. Cases have been reported in cities such as Philadelphia (9), Camden, Wilmington, northern Virginia, and Atlanta.
On the West Coast, both Los Angeles and San Diego reported their first measles cases in February 2024.
From a national perspective, the Centers for Disease Control and Prevention (CDC) reported on January 25, 2024, that four U.S. jurisdictions reported a total of 9 measles cases this year.
The CDC says most measles cases in the U.S. are related to international travelers.
Globally, 64% more measles cases (534,672) were confirmed in 2023 than in 2022.
Since measles is a vaccine-preventable disease, the CDC encourages non-vaccinated or under-vaccinated persons to speak with a healthcare provider about the vaccination options.
The CDC recently published an updated measles vaccination schedule. Various measles vaccines are offered at most pharmacies in the U.S., with financial support from public and private insurance.
According to new data posted by the National Center for Health Statistics (NCHS) Mortality Surveillance, 0.9% of all deaths in the U.S. that occurred during the week ending January 27, 2024 (Week 4), were due to influenza.
On February 2, 2024, the U.S. CDC stated this percentage decreased by ≥ 0.1 compared to Week 3.
During week 1, with about 100% of the data tabulated, there were 703 influenza-related deaths reported by the NCHS.
From October 2, 2022, to September 9, 2023, 9,697 (4%) deaths were listed as influenza.
Additionally, the CDC reported eight influenza-associated pediatric deaths during the 2023-2024 season, which were reported to the CDC during Week 4. A total of 65 influenza-associated pediatric deaths have occurred this flu season and have been reported by the CDC.
Last flu season, there were 178 influenza-associated pediatric fatalities. The mean age at death was about seven years, and 83% of these children were not fully vaccinated.
The CDC continues to recommend annual flu shots (egg, cell, or nasal-based) for most people and booster vaccinations for some people after conferring with a healthcare provider.
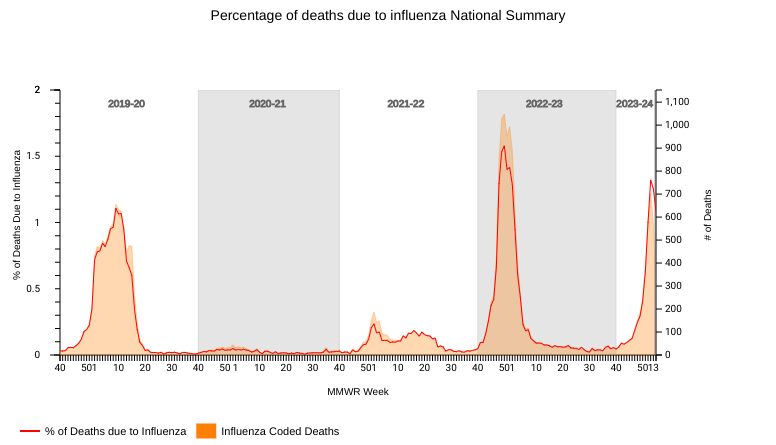
With Merck & Co. Inc.'s recent announcement, health leaders can expect a decrease in human papillomavirus (HPV) cancers in the coming years.
On February 1, 2024, Merck reported that GARDASIL / GARDASIL 9 HPV vaccine sales increased by at least 29% to reach $8.9 billion. This sales growth was due to strong global demand, particularly in China, and public-sector vaccine buying patterns in the U.S.
Robert M. Davis, chairman and chief executive officer of Merck, commented in a press release, "We also made investments of approximately $30 billion in research and development in our ongoing effort to discover, develop, and collaborate to propel the next generation of impactful innovations."
According to the U.S. CDC, getting an HPV vaccine for your child is the best way to protect them against certain types of cancer later in life.
Sexually transmitted cancers usually take years to develop after a person gets HPV. There is no way to know who will develop cancer or other health problems from HPV.
HPV can cause cancers of the cervix, vagina, and vulva in women, penis in men, and anus and throat in both men and women, says the CDC.
While Merck's GARDASIL 9® vaccine is a global market leader in 2024, approved vaccines are currently produced in India and China, with several next-generation vaccine candidates in development to address these vaccine-preventable cancers.
Malaria continues to be the most significant cause of death in young African children, with over 600,000 deaths globally each year. A recently approved malaria vaccine has been proven safe and effective to reduce this global health risk.
The Lancet today published peer-reviewed results from a Phase 3 efficacy clinical trial of the R21/Matrix-M™ malaria vaccine.
The publication reported on February 1, 2024, the following findings:
- Efficacy of 75% when administered before the high transmission season: In areas with highly seasonal malaria transmission (where malaria transmission is primarily limited to 4 or 5 months per year), the R21/Matrix-M vaccine was shown to reduce symptomatic cases of malaria by 75% during the 12 months following a 3-dose series.
- Efficacy of 68% when administered in an age-based schedule in regions where malaria is present perennially during the 12 months following the first three doses.
- Significantly increased immune responses to the R21/Matrix-M vaccine and slightly higher vaccine efficacy were observed in 5-17-month-olds supporting planned vaccine deployment initially from 5 months of age in young African children.
- The most common adverse events with the vaccine were fever (47%) and injection site pain (19%).
John Jacobs, Novavax President and CEO, commented in a press release, "Approximately 1,300 children die from malaria every day, a staggering statistic for a preventable disease."
"The R21/Matrix-M Phase 3 efficacy data published in The Lancet reinforce the potential of R21/Matrix-M vaccine to protect children against this disease."
"We are proud of the role of Novavax's patented saponin-based Matrix-M adjuvant, which has been demonstrated to enhance the immune response, in the outcome of this clinical trial and are eager to see the realized impact of the vaccine when it is rolled out globally."
The study was conducted across multiple sites in four African countries with 4,800 children aged 5-36 months. Data from this trial served as the basis for the World Health Organization's (WHO) recent prequalification of the R21/Matrix-M vaccine, paving the way for a global rollout expected to commence in mid-2024 by Serum Institute of India.
Ghana, Nigeria, Burkina Faso, and regulators in other countries have licensed the vaccine.
Beginning in February 2024, about 25 million R21/Matrix-M vaccine doses will become available. The availability of the R21/Matrix-M vaccine is expected to help close the gap in the vast demand for malaria vaccine doses to protect children against the disease.
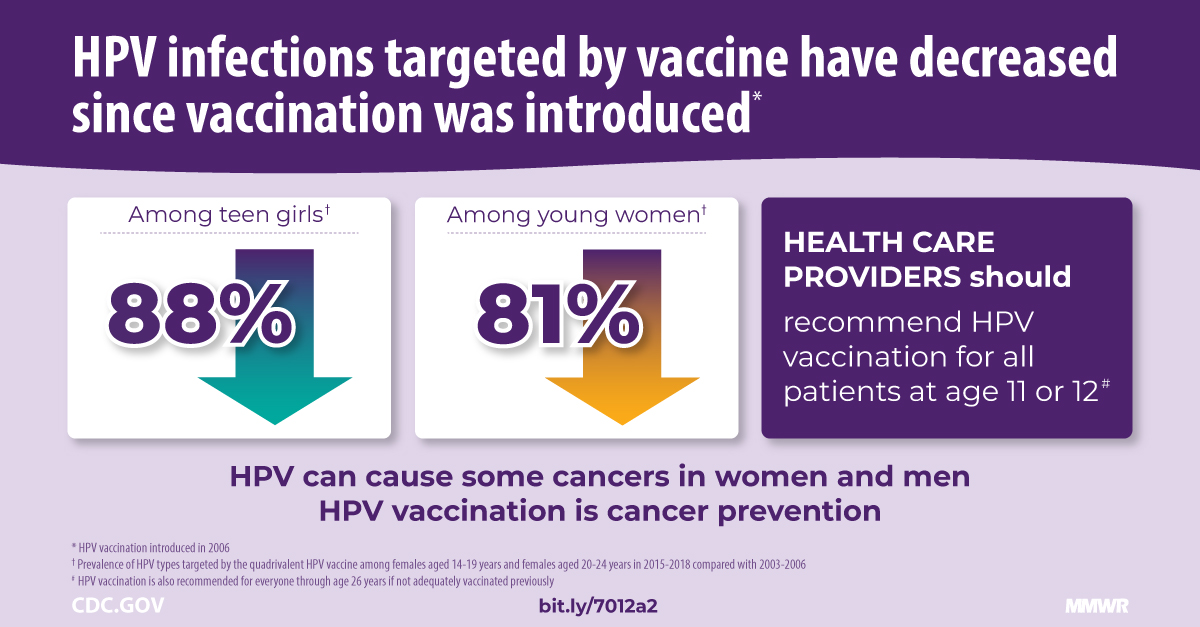
This innovative vaccine contains Novavax Inc.'s saponin-based Matrix-M™ adjuvant.
The Matrix-M adjuvant comes from saponins, naturally occurring compounds in the bark of the Quillaja saponaria (Soapbark) tree. Saponins have a long history of being used for their medicinal properties. The U.S. Food and Drug Administration has approved a vaccine containing another saponin‐based adjuvant.
An Original Article by the New England Journal of Medicine today stated that in 2024, there is an unmet global need for a dengue vaccine that offers protection from all four virus types in a single dose across a wide age range, regardless of dengue serostatus at baseline.
Published on February 1, 2024, this article concluded a phase 3 clinical trial found a single dose of Butantan–Dengue Vaccine (Butantan-DV) prevented symptomatic DENV-1 and DENV-2, regardless of dengue serostatus at baseline, through 2 years of follow-up.
Over the 2-year follow-up, vaccine efficacy against any DENV serotype was 79.6% (95% CI, 70.0 to 86.3).
Secondary endpoints of serotype-specific vaccine efficacy were 89.5% (95% CI, 78.7 to 95.0) against DENV-1 and 69.6% (95% CI, 50.8 to 81.5) against DENV-2.
Regarding baseline dengue serostatus, vaccine efficacy against any serotype was 73.6% (95% CI, 57.6 to 83.7) among participants without evidence of previous dengue exposure (7516 participants) and 89.2% (95% CI, 77.6 to 95.6) among those with evidence of prior dengue exposure (8017 participants).
Butantan-DV is a live, attenuated, tetravalent dengue vaccine candidate composed of vaccine viruses representing all four DENV serotypes analogous to the TV003 formulation developed by the U.S. National Institutes of Health.
Currently, two dengue vaccines are being used by various countries confronted by outbreaks.
GlobalData confirmed today that Beyfortus™ (nirsevimab), a long-acting monoclonal antibody (mAb), has been approved in China for the prevention of respiratory syncytial virus (RSV) lower respiratory tract infection (LRTI) in neonates and infants entering or during their first RSV season.
With the first approved preventive option for RSV, AstraZeneca and Sanofi's Beyfortus will dominate the market in China, says GlobalData, a leading data and analytics company.
GlobalData's RSV Forecast in Asia-Pacific Markets (India, Urban China, Australia, South Korea, and Japan) to 2028 reveals that Urban China will lead the Asia-Pacific market for RSV in 2028, accounting for 34.8% of the overall market size.
Nelluri Geetha, Pharma Analyst at GlobalData, commented in a press release on January 31, 2024, "RSV infection is a leading cause of viral lower respiratory tract infections, with a higher rate seen in children than adults. RSV infection occurs most commonly in children below six months of age in China."
"Beyfortus is the first approved drug for RSV in a broad infant population, which includes healthy term, late preterm, and preterm infants, as well as infants with specific health conditions that make them vulnerable to severe RSV disease."
"Hence, the approval addresses an urgent need for novel prophylactic treatment options for the pediatric population in China."
Geetha concludes: "Beyfortus is the only preventive option for RSV in the infant population, meaning that the drug will continue to dominate the Chinese market shortly."
"However, competition may intensify over the long term as other drugs are in late-stage development for the pediatric population in this market. These include Merck & Co's clesrovimab and Zhuhai Trinomab Biotechnology's TNM-001 in Phase III development."
"These are mAbs in Phase III development for the prevention of RSV among pediatric patients."
As of February 1, 2024, Beyfortus is available in the U.S., U.K., and European markets for the 2024 RSV season. In 2023, Beyfortus sales reached €547 million in 2023.
Vaxcyte, Inc., a clinical-stage vaccine innovation company engineering high-fidelity vaccines to protect humankind from the consequences of bacterial diseases, announced today the pricing of an underwritten public offering of common stock and pre-funded warrants.
According to the Company's press release on January 30, 2024, this is potentially a $750 Million public offering.
Vaxcyte is developing broad-spectrum conjugate and novel protein vaccines to prevent or treat bacterial infectious diseases.
Vaxcyte is re-engineering how highly complex vaccines are made through modern synthetic techniques, including advanced chemistry and the XpressCF™ cell-free protein synthesis platform, exclusively licensed from Sutro Biopharma, Inc.
Unlike conventional cell-based approaches, the Company's system for producing difficult-to-make proteins and antigens is intended to accelerate its ability to efficiently create and deliver high-fidelity vaccines with enhanced immunological benefits.
Vaxcyte's lead vaccine candidate, VAX-24, is a Phase 3-ready 24-valent, broad-spectrum, carrier-sparing pneumococcal conjugate vaccine (PCV) being developed to prevent invasive pneumococcal disease.
VAX-31, the Company's next-generation 31-valent PCV, is the broadest-spectrum PCV candidate in the clinic today.