Search API
The European Centre for Disease Prevention and Control (ECDC) recently confirmed sporadic human cases of avian influenza A (H9N2) have been observed in 2023, mainly in young children.
As of June 12, 2023, one new human infection with H9N2 was reported in Sichuan province, China.
And since 1998, a total of 125 laboratory-confirmed cases, including two deaths, of human infection with H9N2 viruses have been reported in eight countries: China (112), Egypt (4), Bangladesh (3), Cambodia (2), Oman (1), Pakistan (1), India (1), and Senegal (1).
Over the past year, various humans have been infected with different avian influenza viruses.
According to the Centers for Disease Control and Prevention, Avian influenza or bird flu is caused by infection with avian (bird) influenza (flu) Type A viruses.
And the Highly Pathogenic Avian Influenza (HPAI) strain of the H5N avian flu is currently spreading in the U.S.
In the U.S., the government has already authorized one bird-flu vaccine and invested in developing other vaccine candidates.
SK bioscience today announced the company has received the WHO Emergency Use Listing (EUL) of its COVID-19 vaccine, SKYCovione™ (SKYCovion™, GBP510).
This COVID-19 vaccine was developed with the Institute for Protein Design (IPD) at the University of Washington SCHOOL OF MEDICINE and uses GSK's pandemic adjuvant.
SKYCovione is also the world's first medicine developed using computational protein design, an approach that uses Rosetta software to engineer protein structures with enough precision to place individual atoms exactly where desired.
Jaeyong Ahn, CEO of SK bioscience, commented in a press release on June 19, 2023, "Based on the immunogenicity and safety profile, SKYCovione has become the first Korean vaccine to be granted to the WHO EUL."
"We will be committed to developing more vaccines not just to strengthen Korea's vaccine sovereignty but also to enable equitable access to the vaccine."
The development of SKYCovione has been supported by funding from the Bill & Melinda Gates Foundation and Coalition for Epidemic Preparedness Innovations with support from the European Horizon 2020 Programme.
SKYCovione (known as SKYCovion in the UK) was approved by the Medicines and Healthcare products Regulatory Agency for adults in May 2023.
SKYCovione is a self-assembled nanoparticle vaccine targeting the receptor binding domain of the SARS-CoV-2 Spike protein for SARS-CoV-2.
The vaccine can be stored between 2-8 °C, making it suitable for use in countries where ultra-low cold chain storage facilities are unavailable. The ease of distribution helps to achieve greater access to vaccines in low-income countries.
Other COVID-19 vaccine news is posted by Precision Vaccinations.

UK Health Security Agency (UKHSA) announced today London had reported 11 new mpox cases within the past few weeks.
Most of these cases were in unvaccinated individuals. However, three were in those who had only received one dose of Bavarian Nordic's JYNNEOS® (MVA-BN) vaccine.
In response to London's mpox outbreak, the UKHSA vaccinations would be offer in London to qualifying men beyond July 2023.
After the end of July, people who regularly travel to London or abroad to have sex and are eligible for the JYNNEOS vaccine are empowered to schedule a London-based appointment using the vaccine finder.
Dr. Claire Dewsnap, President of the British Association for Sexual Health and HIV, commented in a related press release, "We strongly encourage all those eligible to book an appointment to receive a mpox vaccination, especially given the worrying recent spike in cases."
"One dose of the vaccine protects against the virus, and the second dose can further prevent severe symptoms and transmission."
"This is particularly important as we move into the summer months, during which festivals and events are more common, increasing the likelihood for people to have multiple sexual partners."
As of June 16, 2023, 21 mpox cases have been reported in the UK.
Other mpox outbreaks are posted by Vax-Before-Travel.

Novavax, Inc. today announced it participated in the U.S. Food and Drug Administration (FDA) Vaccines and Related Biological Products Advisory Committee's (VRBPAC) meeting.
This VRBPAC meeting resulted in a unanimous vote recommending updating the current COVID vaccine composition to a monovalent XBB-lineage.
"Novavax expects to be ready for the commercial delivery of a protein-based monovalent XBB COVID vaccine this fall in line with today's VRBPAC recommendation," said John C. Jacobs, President, and Chief Executive Officer, Novavax, in a press release on June 15, 2023.
"In partnership with regulators and public health authorities, Novavax has been developing and manufacturing this vaccine candidate, and now that we are nearing harmonization on guidance from the FDA, the World Health Organization, and European Medicines Agency, we believe we are in a better position to offer an alternative vaccine choice for individuals worldwide."
Novavax presented data at the VRBPAC meeting that supports the recommendation to vaccinate this fall (2023) with a monovalent XBB strain.
Novavax data showed that its XBB.1.5 COVID vaccine candidate induced functional immune responses for XBB.1.5, XBB.1.16, and XBB.2.3 variants, indicating a broad response that could be applicable for forward-drift variants.
The journal Vaccine published study results on June 2, 2023 that found correlates of protection imply a fourth (5 µg SARS-CoV-2 recombinant spike protein + 50 µg Matrix-M™ adjuvant) post-boost efficacy of ≥ 82% for Omicron variants and did not increase local/systemic reactogenicity in those aged 18–84 years.
Novavax COVID-19 vaccine brands include Nuvaxovid, CovoVax, NVX-CoV2373, and TAK-019, and are available in about 40 markets globally.

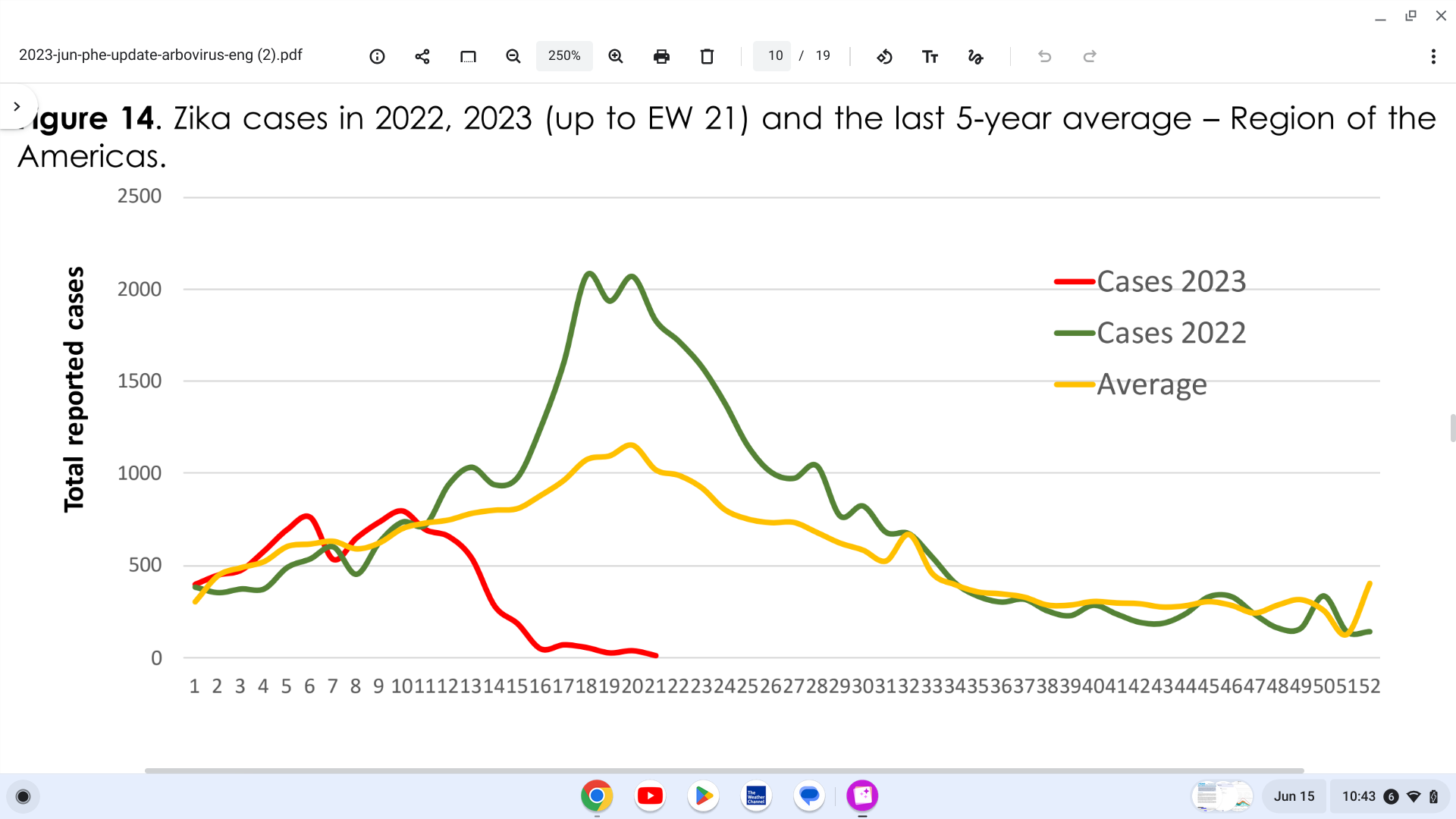
The Pan American Health Organization (PAHO) recently confirmed local transmission of Zika in countries and territories in the Region of the Americas.
As of June 10, 2023, up to EW 21, 8,758 Zika cases were reported in the Region of the Americas.
The highest proportion of Zika cases were reported in Brazil (7,352), followed by Bolivia and Belize.
Furthermore, per Brazilian authorities, over 1,638 babies have been born with microcephaly-related health issues since 2014.
Previously, the U.S. CDC stated that because the mosquitoes that spread the Zika virus are found throughout Puerto Rico, people living on the island who have not already been infected are at risk for infection.
Puerto Rico's Weekly Arboviral Diseases Report #22 shows 26 probable Zika cases as of June 13, 2023.
As of June 15, 2023, the U.S. FDA has not approved a Zika vaccine candidate.