Search API
The Joint Committee on Vaccination and Immunisation (JCVI) today announced its advice to the U.K. Government for the COVID-19 vaccine programme in spring 2024.
Similar to recent campaigns, the JCVI's advice issued on February 7, 2024, is to offer an updated COVID-19 vaccine to those at high risk of serious disease and who are, therefore, most likely to benefit from vaccination.
JCVI advises the following groups be offered vaccination this spring:
- Adults aged 75 years and over. COVID-19 vaccine uptake for the 2023 spring program for those 75 years and over was 67.5%.
- Residents in a care home for older adults.
- Individuals aged six months and over who are immunosuppressed. This follows updated advice in April 2023 on COVID-19 vaccination of children aged six months to 4 years in a clinical risk group.
Professor Wei Shen Lim, Chair of COVID-19 immunization on the JCVI, said in a press release, "The COVID-19 spring program will continue to focus on those at greatest risk of getting seriously ill, who will benefit the most from a further vaccine dose."
"It is important that everyone eligible takes up the offer this spring."
Utilizing a deployment cost of £25 per vaccine, the non-standard cost-effectiveness assessment for booster vaccination in spring 2024 indicated that vaccination was likely cost-effective when offered to most people over 65 within the assumptions describing the most plausible projected scenario.
In addition to mRNA vaccines, Novavax Matrix-M adjuvanted COVID-19 vaccine (Nuvaxovid) and HIPRA bivalent COVID-19 vaccine (Bimervax) may be used as a booster dose for certain persons in 2024.
As of February 8, 2024, 13 COVID-19 vaccines have been granted Emergency Use Listing by the World Health Organization. Recent additions include SKYCovione™ and CORBEVAX®.

When the U.S. government approved the first two respiratory syncytial virus (RSV) vaccines in 2023, the indication was for people 60 years and older to prevent lower respiratory tract disease (LRTD) caused by RSV.
Based on recent filings, it appears adults 50 years and older may have access to these important vaccines in time for the next RSV season.
Increasing access to RSV vaccines is viewed as a global public health goal. As of early February 2024, the percentage of U.S. adults age 60+ who report receiving an RSV vaccine this season is 20.8%.
GSK plc announced on February 6, 2024, that the U.S. Food and Drug Administration (FDA) has accepted under Priority Review an application to extend the indication of AREXVY™ adjuvanted RSV vaccine to adults aged 50-59 who are at increased risk for RSV disease.
The Prescription Drug User Fee Act date, the FDA action date for their regulatory decision, is June 7, 2024.
Previously, on December 12, 2023, Japan's Ministry of Health, Labour, and Welfare accepted a similar application for AREXVY.
If approved, AREXVY would be the first RSV vaccine available to help protect this population during the 2024-2025 RSV season.
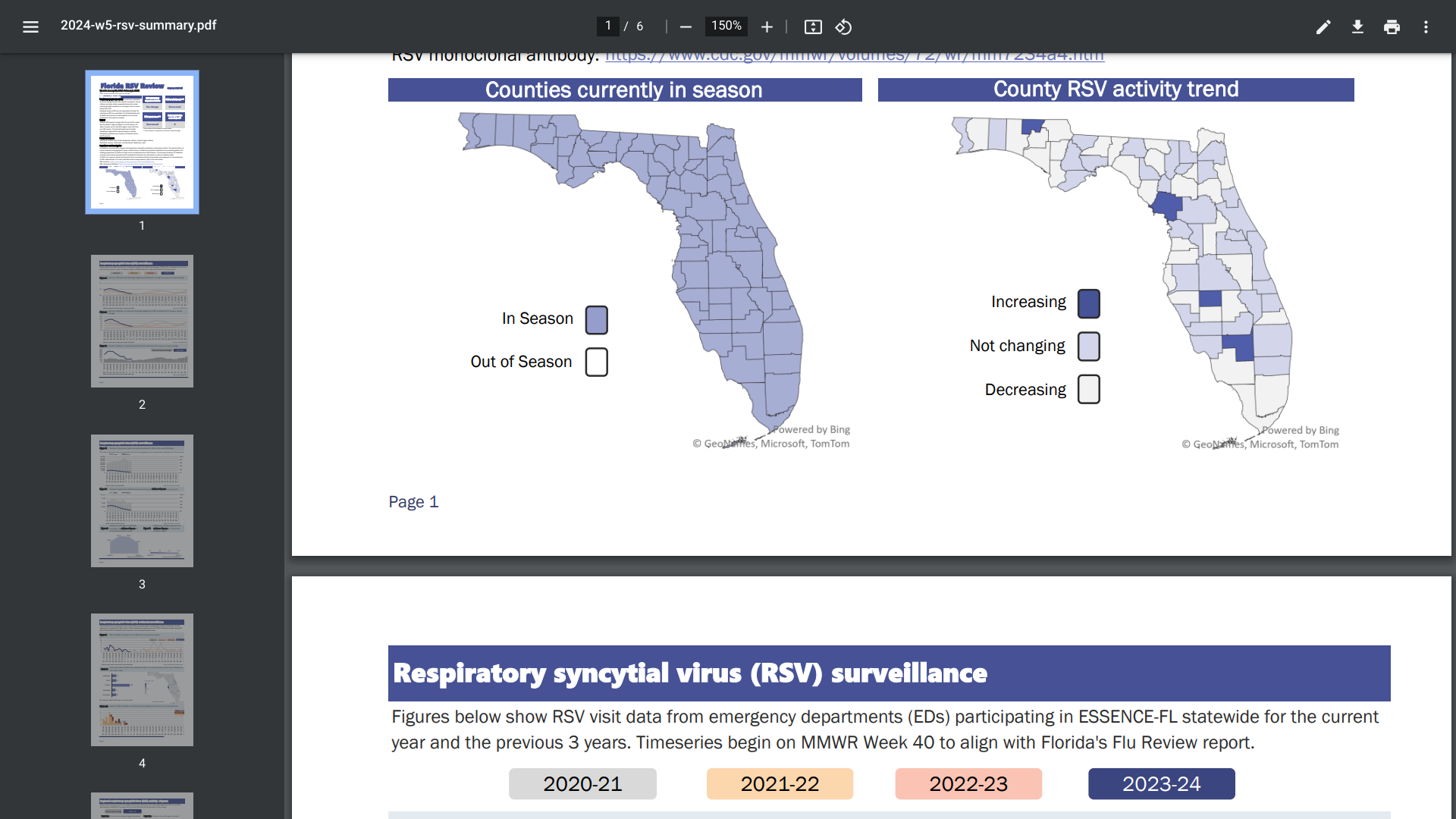
As of February 2024, RSV activity was stable or decreased in North America, and most European reporting countries reported the World Health Organization Influenza Update N° 462.
In the U.S., the Centers for Disease Control and Prevention's RSV detection graphs display the 5-week moving average, recently indicating decreased cases in certain states.
The Weekly Influenza Vaccination Dashboard uses various data sources to share preliminary weekly flu vaccination data, including coverage estimates.
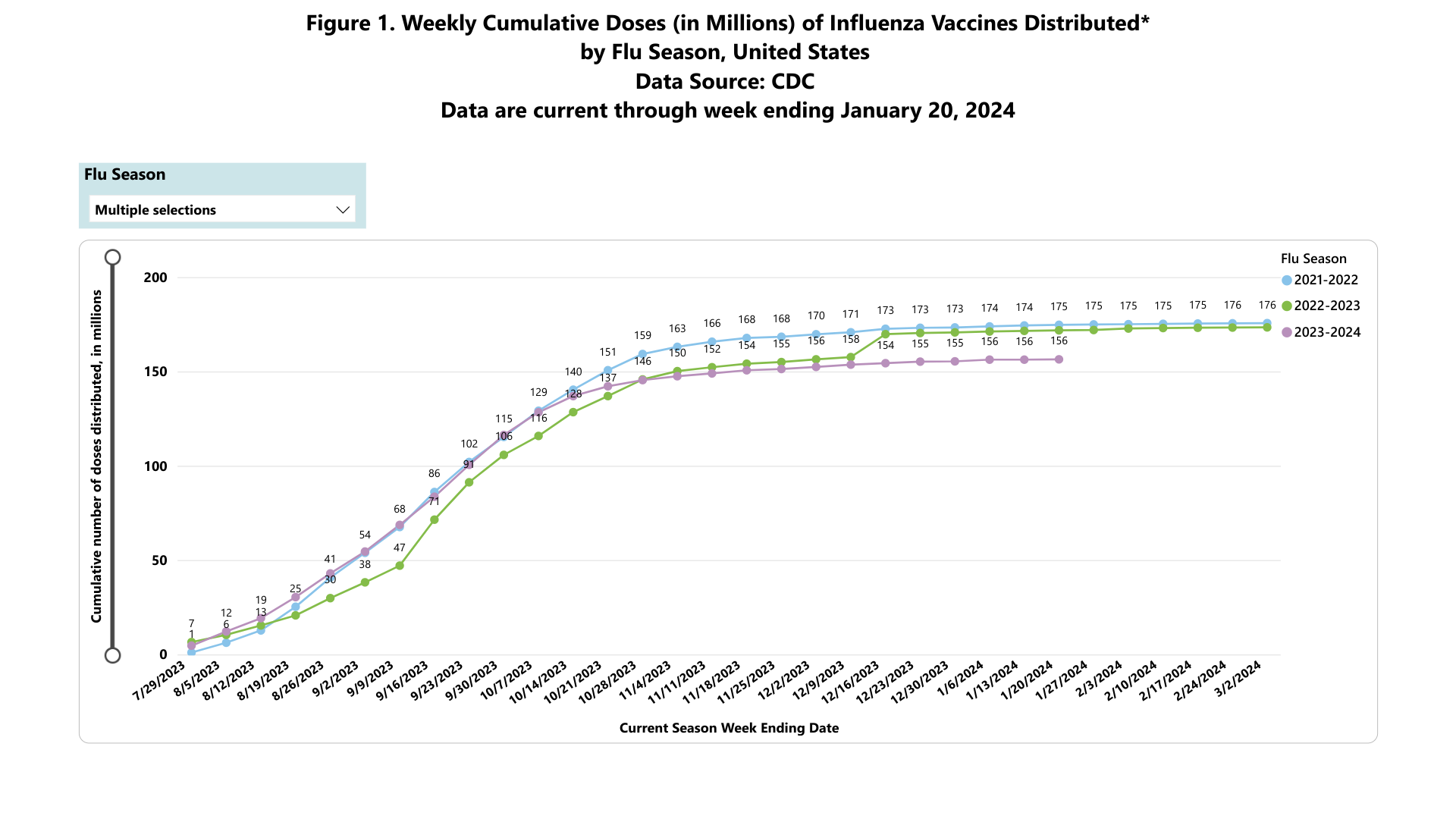
According to recent data published by the U.S. Centers for Disease Control and Prevention (CDC), influenza vaccination rates have been trending lower over the past three flu seasons.
As of January 20, 2024, the CDC's data indicates about 156 million influenza vaccines have been distributed during the 2023-2024 season. However, this data may change as the flu season progresses.
During the 2021-2022 season, 176 million vaccines were distributed in the U.S.
From a demographic perspective, the CDC reports the flu shot coverage estimates for the 2023-24 season are as follows:
Coverage for all children is 3.5% lower this season than last season (47.8% compared with 51.3%). Coverage this season so far is 9.6 percentage points lower compared with pre-pandemic coverage at the same time in January 2020 (57.4%).
For pregnant women, coverage at the end of December 2023 (35.7%) is 3.3% lower compared with coverage at the end of December 2022 (39%).
National coverage for all U.S. adults is 47%. For adults 65 years and older, an estimated 43.4% of Medicare fee-for-service beneficiaries have been vaccinated this season.
And flu shot coverage among states and D.C. ranges from 36.8% to 62.3%.
Last week, the CDC stated seasonal influenza activity remains elevated nationally, with increases in some parts of the country. And it recommends that everyone six months and older get an annual flu vaccine as long as influenza viruses are spreading.
CSL and Arcturus Therapeutics today announced the results of a follow-up analysis of a Phase 3 study evaluating a booster dose of ARCT-154, the world's first approved self-amplifying messenger RNA (sa-mRNA) COVID-19 vaccine, compared to a conventional mRNA COVID-19 vaccine.
The new analysis at six months post-vaccination shows that ARCT-154 induces a longer immune response than Comirnaty® for both the original Wuhan strain and Omicron BA.4/5 variant and an advantage in antibody persistence.
ARCT-154 was administered at one-sixth the dose of Comirnaty® (5 μg vs 30 μg, respectively).
"These results further support sa-mRNA's differentiating attribute to provide prolonged protection against COVID-19 at lower doses," said Jonathan Edelman, M.D., Senior Vice President, Vaccines Innovation Unit, CSL, in a press release on February 5, 2024.
"Protecting the global public from viral respiratory diseases remains a top priority for us, and we look forward to continuing to collect and share data at the twelve-month post-booster mark."
Unlike standard mRNA vaccines, these companies commented that self-amplifying mRNA vaccines instruct the body to make more mRNA and protein to boost the immune response.

Valneva SE announced today that it recently sold the Priority Review Voucher (PRV) it received from the U.S. Food and Drug Administration (FDA) for $103 million.
The Company was awarded a tropical disease PRV in November 2023 following U.S. FDA approval of IXCHIQ®, Valneva's single-dose, live-attenuated vaccine indicated for preventing disease caused by chikungunya virus.
Under the Tropical Disease Priority Review Voucher Program, the FDA awards priority review vouchers to sponsors of tropical disease product applications that meet certain criteria. The program is intended to encourage the development of new drugs and biologics to prevent and treat tropical diseases.
PRVs can be redeemed to receive priority review of a subsequent marketing application for a different product, sold or transferred.
In a press release, Thomas Lingelbach, Chief Executive Officer of Valneva, commented, "This non-dilutive capital provides an important source of additional funding to advance the continued development of our clinical pipeline."
"As shown with the recent approval of our chikungunya vaccine, we remain committed to growing our portfolio of vaccines addressing unmet medical needs which have the potential to transform people's lives."
With the FDA's approval in 2023, IXCHIQ became the world's first licensed chikungunya vaccine to address this unmet medical need.

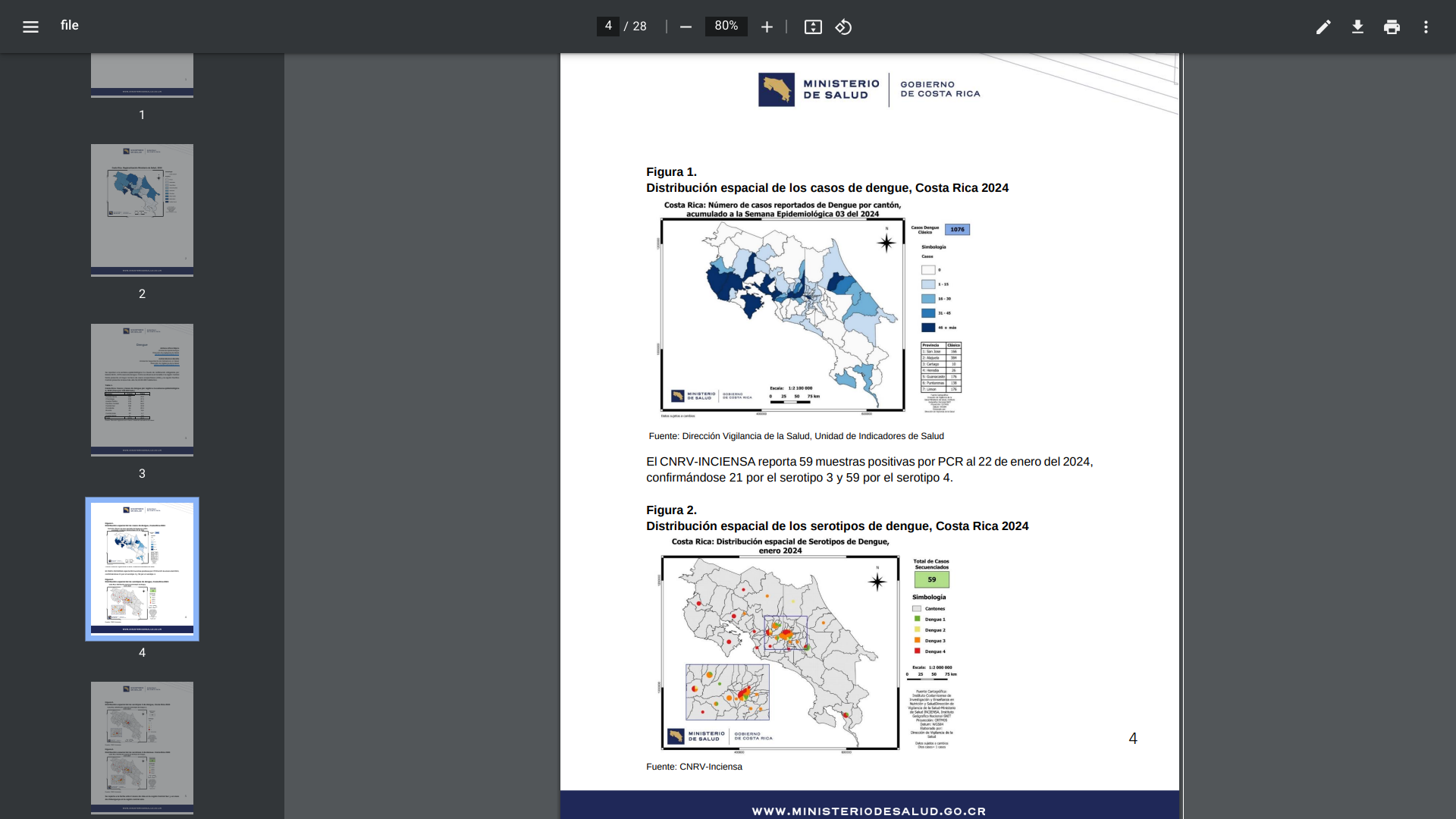
The Central American country of Costa Rica has been battling an outbreak of dengue fever, both classic and hemorrhagic, for the past few years. This mosquito-transmitted virus continues to impact Costa Rica residents and visitors in 2024.
On February 2, 2024, the Costa Rica Health Ministry reported that 1,076 dengue cases led by Central Norte have been confirmed this year.
Throughout 2023, there were about 24,914 dengue cases reported, a significant increase from the 7,485 patients in 2022.
Costa Rica is not alone in the Region of the Americas with the acceleration of dengue cases.
The Pan American Health Organization (PAHO) recently issued various dengue Risk Assessments that said dengue is endemic in most countries of South America, Central America, and the Caribbean. In 2023, the Americas experienced a 57% increase in dengue cases compared to 2022.
As of December 2023, the PAHO issued a Situation Report that assessed the risk of dengue outbreaks in the Americas as high at the regional level due to the widespread distribution of the Aedes spp. Mosquitoes.
In the U.S., the Centers for Disease Control and Prevention (CDC) has issued Travel Health Notices regarding dengue outbreaks in the Americas. The CDC reported on January 3, 2024, that there were 2,343 dengue cases reported by 52 U.S. jurisdictions during 2023.
The CDC says dengue is endemic in the U.S. territories of Puerto Rico, American Samoa, the U.S. Virgin Islands, the Federated States of Micronesia, the Republic of Marshall Islands, and the Republic of Palau.
Dengue is a vaccine-preventable disease, with two vaccines currently in use in the Americas and several dengue vaccine candidates in development in 2024.