Search API
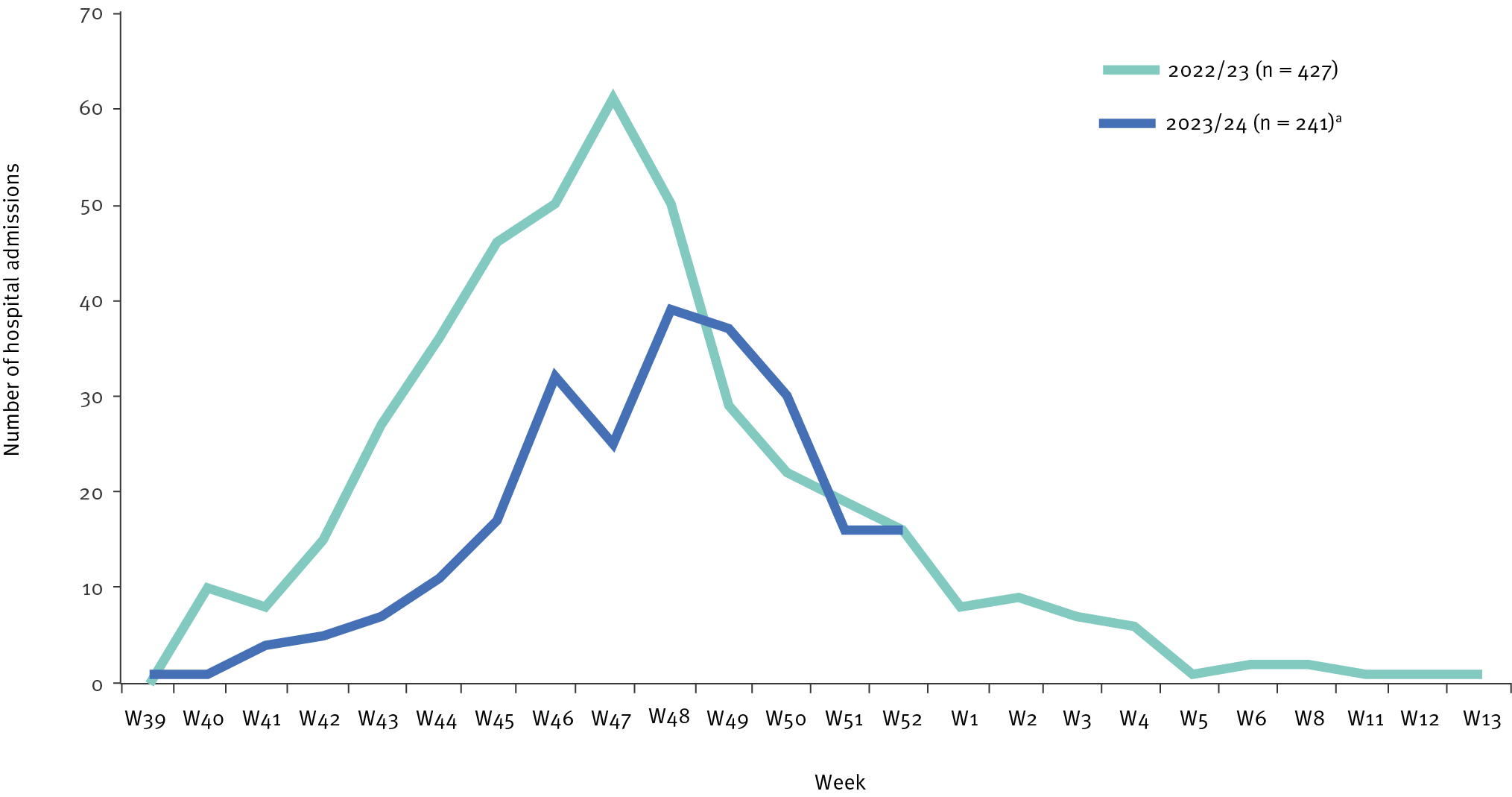
The journal Eurosurveillance Rapid Communication published a study on January 25, 2024, that concluded Beyfortus™ (Nirsevimab) was about 69% effective at preventing pediatric respiratory syncytial virus (RSV) hospitalizations in infants.
In the context of moderate to high immunization coverage (84%) among neonates, this study provides early real-world evidence of nirsevimab immunization protecting infants from severe RSV disease in Luxembourg.
In 2023, 241 children under five years of age were hospitalized with a laboratory-confirmed RSV infection, compared with 389 cases in 2022, representing decreases of 38% (389 vs. 241) in cases under five years of age and 69% (232 vs. 72) in cases of infants under six months old.
The researchers concluded, 'Our study shows the impact of nirsevimab in mitigating severe RSV disease among infants during the first RSV season following the national implementation of passive immunization achieving high coverage in Luxembourg. There was a significant increase in the age of hospitalized children, and most severe RSV-related hospitalizations occurred in non-immunized children."
In previous clinical trials. nirsevimab showed between 74% and 86% efficacy against medically-attended lower respiratory tract infections caused by RSV in healthy infants.
As of February 2024, Beyfortus's availability in the U.S. has significantly improved.
YS Biopharma Co., Ltd. today announced that it has entered into a share purchase agreement with an institutional investor for a private placement for an aggregate of US$40 million in proceeds.
As of February 9, 2024, YS Biopharma has developed a proprietary PIKA® immunomodulating technology platform and a new generation of preventive and therapeutic biologics targeting Rabies and other virus infections.
According to the World Health Organization, Rabies is a vaccine-preventable viral disease found in more than 150 countries and territories.
The PIKA rabies vaccine candidate is a lyophilized human-use rabies vaccine composed of cell culture-derived rabies antigen mixed with PIKA adjuvant, which acts as a TLR3 agonist. It is designed to induce accelerated and strong cellular immunity and rapidly stimulate the body to produce a higher humoral immune response.
And its accelerated onset of immune response allows a three-visit, one-week regimen superior to the currently available vaccine with a five-visit, one-month or three-visit, three-week regimen, which shortens the treatment period by two to three weeks.
The clinical studies to date have shown that PIKA rabies vaccine can achieve protective level of neutralizing antibodies as early as seven days post vaccination and elicit more robust immunogenic response compared to that of the control arm vaccine, which is a widely used commercially available vaccine.
On October 31, 2023, the company completed enrollment in its Phase 3 clinical trial, which will assess the safety, immunogenicity, and lot-to-lot consistency of the PIKA Rabies Vaccine.
On June 1, 2023, the Food and Drug Administration of the Philippines granted Phase 3 clinical trial approval, and on May 16, 2023, Pakistan issued study approval.
In the U.S., dog control programs were initiated in the 1940s. Since then, routine rabies vaccination eliminated the canine rabies virus variant from circulation by 2008.
As of 2024, bats are the leading cause of rabies deaths in people in all 49 continental U.S. states. Currently, several rabies vaccines for people, such as Chirorab®, are available worldwide.

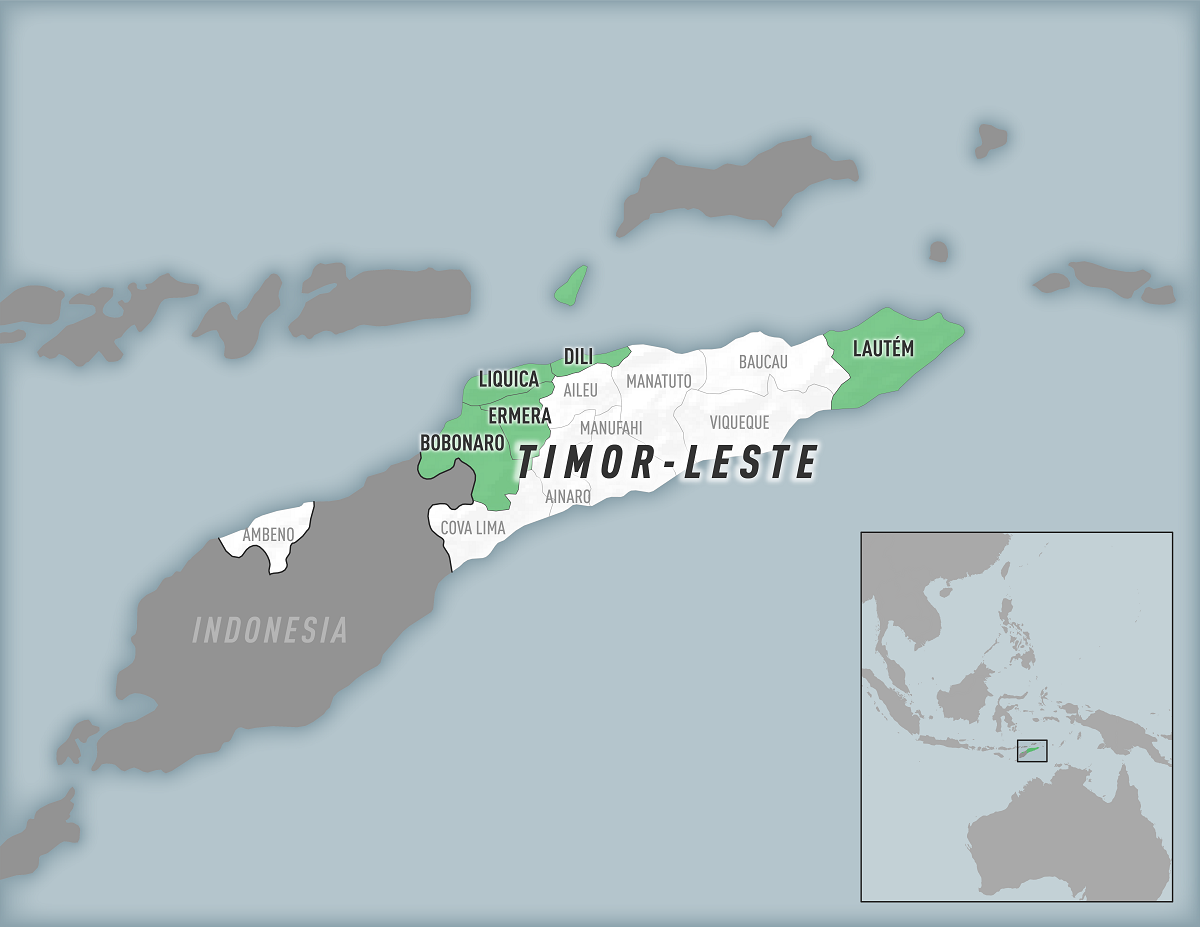
The U.S. Centers for Disease Control and Prevention (CDC) issued a Travel Health Notice today confirming a chikungunya virus outbreak is impacting several municipalities in Timor-Leste, also known as East Timor.
Located about 2,000 miles north of Australia, local media recently reported about 183 chikungunya cases.
Timor-Leste's Vice-Minister of Health previously noted that "since the Restoration of Timor-Leste's Independence, we have faced many problems, including several diseases. Being a tropical country, our country is considered an endemic zone for several infectious diseases (malaria, dengue) caused by vectors (mosquitoes)."
On February 8, 2024, the CDC said people could avoid this mosquito-transmitted disease by using insect repellent, wearing long-sleeved shirts and pants, staying in places with air conditioning, or using window and door screens.
If infected, you should seek medical care if you develop fever, joint pain, headache, muscle pain, joint swelling, or rash during or after travel.
However, chikungunya can be fatal.
A recent study published by the Lancet Infectious Diseases journal found chikungunya disease is associated with an increased risk of death for up to 84 days after symptom onset.
Furthermore, the CDC stated in this Level 2 - Practice Enhanced Precautions Notice that if you are pregnant, reconsider travel to Timore-Leste, mainly if you are close to delivering your baby. Mothers infected around the time of delivery can pass the virus to their baby before or during delivery.
Newborns infected this way or by mosquito bites are at risk for severe illness, including poor long-term outcomes.
While the U.S. FDA recently approved Valneva SE's IXCHIQ® Chikungunya Vaccine, the CDC has yet to authorize its use in the U.S.
The CDC's vaccine committee is scheduled to review this vaccine on February 28, 2024. They intend to review proposed policy options for chikungunya vaccine use among U.S. adults traveling abroad.
As of February 2024, several countries have recently confirmed chikungunya outbreaks,
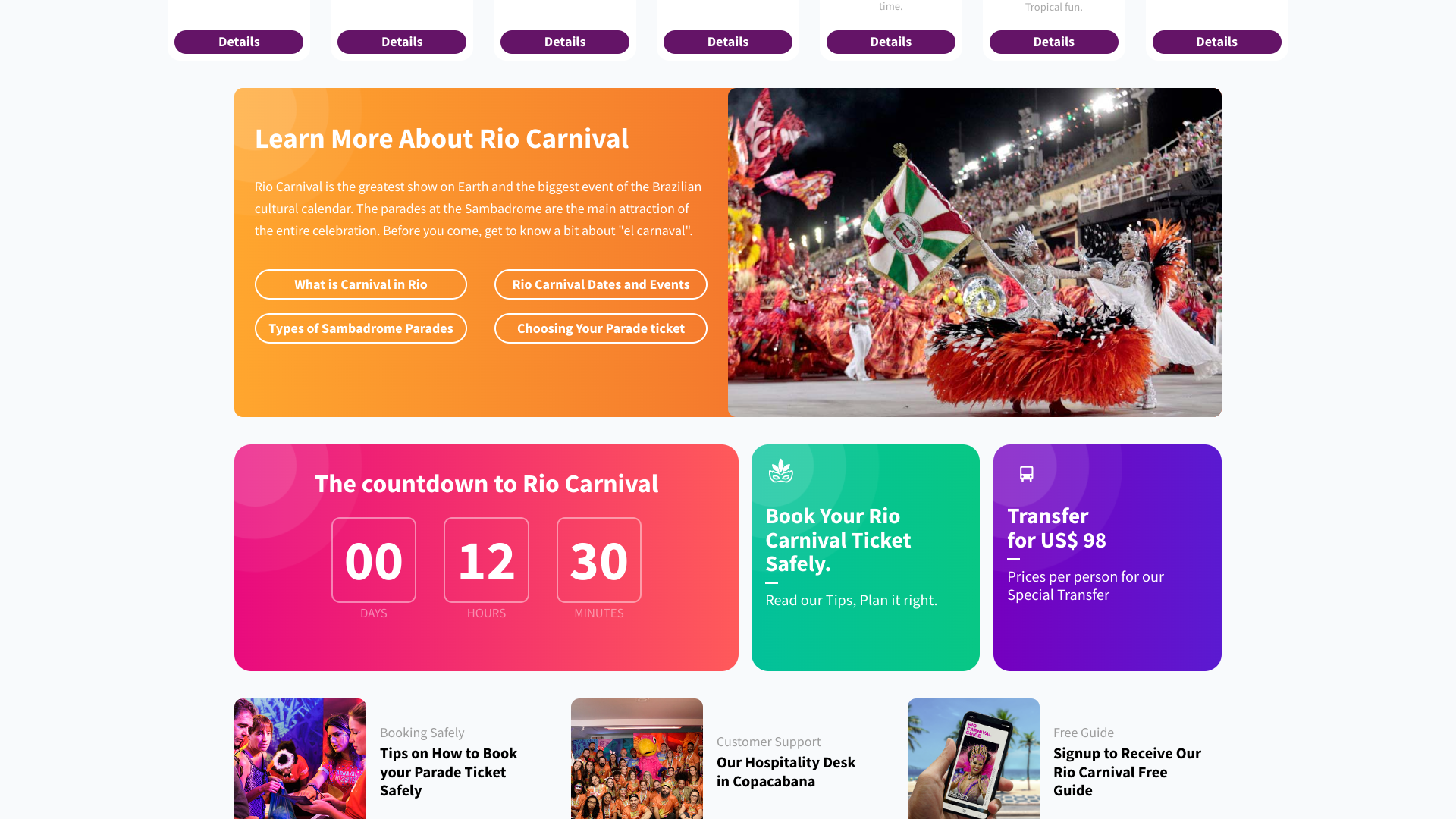
As millions of residents and international travelers celebrate Carnival in Rio de Janeiro today, a mosquito-borne disease may disrupt two million people per day attending the biggest festival in the world.
As of February 9, 2024, the Federative Republic of Brazil reported over 10,000 dengue virus cases, compared to 2.9 million in 2023.
And Rio's Carnival could be a hot-spot for disease transmission.
Dengue is a vaccine-preventable disease, endemic in about 125 countries.
To reduce dengue infections, Brazil's health regulator approved the QDENGA® second-generation vaccine in 2023. Unfortunately, this dengue vaccine has not yet been widely distributed this year.
This means that in addition to festival-related headaches, many Carnival attendees may experience fever, pain behind the eyes, muscle and joint pain, and a blotchy rash.
Furthermore, on rare occasions, dengue can be fatal.
During 2023, a total of 2,050 deaths due to dengue in the Americas resulted in a case fatality rate of 0.049%.
There is good news from the local government.
On February 7, 2024, Brazil announced a National Plan for the Elimination of Socially Determined Diseases. This plan includes increasing vaccine access to prevent various diseases, including dengue.
Numerous countries are reporting measles cases as the global resurgence extends into 2024.
For example, there has been a measles outbreak in England.
The U.K. Health Security Agency (UKHSA) today announced that 118 laboratory-confirmed measles cases were confirmed in England last week, bringing the total number of measles cases to 465 since October 2023.
Of these cases, 71% (329 of 465) have been reported in the West Midlands, 13% in London, and 7% in Yorkshire and The Humber. The remaining cases were reported in other regions of England.
The majority (66%) of these cases are in unvaccinated children under the age of 10.
Dr Vanessa Saliba, UKHSA Consultant Epidemiologist, stated in a press release on February 8, 2024, "MMR vaccine uptake has been falling over the last decade with 10% of children starting school in England not protected."
"Parents should be aware that measles is a nasty illness for most children and, sadly for some, can be very serious and life-changing, but it is completely preventable."
"I strongly urge parents to take up the offer as soon as possible and protect their child now."
According to the U.S. CDC, measles is caused by a highly contagious virus that spreads through the air by direct contact with infectious droplets or by airborne spread when an infected person breathes, coughs, or sneezes.
All international travelers, including young children, should be fully vaccinated against measles, as infected people can spread measles up to four days before and after a rash.
Worldwide, there was a 64% increase in measles cases in 2023 compared to 2022.
As of 2024, the CDC maintains a global Watch-Level 1, Practice Usual Precautions, Travel Health Notice, identifying measles outbreaks in 47 countries.

The Joint Committee on Vaccination and Immunisation (JCVI) today announced its advice to the U.K. Government for the COVID-19 vaccine programme in spring 2024.
Similar to recent campaigns, the JCVI's advice issued on February 7, 2024, is to offer an updated COVID-19 vaccine to those at high risk of serious disease and who are, therefore, most likely to benefit from vaccination.
JCVI advises the following groups be offered vaccination this spring:
- Adults aged 75 years and over. COVID-19 vaccine uptake for the 2023 spring program for those 75 years and over was 67.5%.
- Residents in a care home for older adults.
- Individuals aged six months and over who are immunosuppressed. This follows updated advice in April 2023 on COVID-19 vaccination of children aged six months to 4 years in a clinical risk group.
Professor Wei Shen Lim, Chair of COVID-19 immunization on the JCVI, said in a press release, "The COVID-19 spring program will continue to focus on those at greatest risk of getting seriously ill, who will benefit the most from a further vaccine dose."
"It is important that everyone eligible takes up the offer this spring."
Utilizing a deployment cost of £25 per vaccine, the non-standard cost-effectiveness assessment for booster vaccination in spring 2024 indicated that vaccination was likely cost-effective when offered to most people over 65 within the assumptions describing the most plausible projected scenario.
In addition to mRNA vaccines, Novavax Matrix-M adjuvanted COVID-19 vaccine (Nuvaxovid) and HIPRA bivalent COVID-19 vaccine (Bimervax) may be used as a booster dose for certain persons in 2024.
As of February 8, 2024, 13 COVID-19 vaccines have been granted Emergency Use Listing by the World Health Organization. Recent additions include SKYCovione™ and CORBEVAX®.