Search API
Brii Biosciences Limited today announced that it has entered into agreements with VBI Vaccines, Inc., ensuring expansion and control of future clinical and commercial supplies of BRII-179, a late-stage clinical asset in Brii Bio's HBV functional cure portfolio.
Brii Bio confirmed on February 13, 2024, that it will initially issue a $2.5 million promissory note to VBI. This will eliminate royalty and milestone payments for PreHevbri. The note will increase to $10 million upon meeting specific conditions, securing all of VBI's intellectual properties for BRII-179, with associated payments also eliminated.
In addition, subject to certain approvals, Brii Bio and VBI will work together to transfer the manufacturing technologies of BRII-179 to a site designated by Brii Bio.
Upon completing essential activities relating to such technology transfer, subject to certain potential adjustments, Brii Bio will issue up to an additional $8 million promissory note to VBI.
After satisfaction of certain conditions, Brii Bio will also take control of VBI's Rehovot-based manufacturing facilities for BRII-179 and PreHevbrio™ (PreHevbri®, Sci-B-Vac®) for $10 million cash on or after June 30, 2024, when Brii Bio and VBI plan to enter into supply agreement under which Brii Bio will become VBI's commercial supplier for PreHevbrio and PreHevbri.
Separately, subject to achievement of certain conditions by VBI, Brii Bio will secure an exclusive license to develop and commercialize VBI-1901, VBI's glioblastoma immunotherapeutic candidate, in the Asia Pacific region excluding Japan and issue a $5 million promissory note to VBI. VBI-1901 has received fast-track and orphan drug designations from the U.S. Food and Drug Administration and is conducting a Phase 2b study.
Dr. Zhi Hong, Ph.D., Chairman and Chief Executive Officer of Brii Bio, stated in a press release, "As Brii transitions to late-stage development of HBV programs, a global manufacturing strategy becomes critically important."
"We look forward to working with the biologics manufacturing experts at the Rehovot site and timely integration of our R&D and manufacturing capabilities."
According to the U.S. CDC, Hepatitis B is a vaccine-preventable liver infection caused by the HBV. It is spread when blood, semen, or other body fluids from a person infected with the virus enter the body of someone who is not infected. Not all people newly infected with HBV have symptoms, but for those that do, symptoms can include fatigue, poor appetite, stomach pain, nausea, and jaundice.

GSK plc announced today that the US Food and Drug Administration (FDA) has granted Fast Track designation for bepirovirsen, an investigational antisense oligonucleotide (ASO) for the treatment of chronic hepatitis B (CHB).
GSK said on February 12, 2024, Bepirovirsen is the only single agent in phase III development that has shown the potential to achieve clinically meaningful functional cure response when combined with oral nucleoside/nucleotide analogues (NAs).
The FDA designation was requested based on the potential for bepirovirsen to address an unmet medical need for CHB, a serious and life-threatening condition.
Data from the phase IIb trials B-Clear and B-Sure, which evaluated the efficacy, safety, and durability of the response of bepirovirsen in people with CHB, were submitted to support the application. A confirmatory phase III program, B-Well, is ongoing.
CHB affects nearly 300 million people worldwide, and current treatment options offer a less than 2-8% functional cure rate, which is not clinically meaningful.
Currently, available oral antiviral therapies only suppress the virus and do not directly lower hepatitis B surface antigen, which is essential for a functional cure.
Bepirovirsen is a triple-action investigational antisense oligonucleotide. It is also being investigated as a potential backbone therapy in future sequential regimens to pursue functional cures in a broader population of patients with CHB.
FDA Fast Track designation is intended to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need.
Bepirovirsen (previously known as 'ISIS 505358 or IONIS-HBVRX') was discovered and is jointly developed with Ionis Pharmaceuticals.
GSK is a global biopharma company that aims to unite science, technology, and talent to get ahead of disease together. Find out more at gsk.com. GSK's unedited press release is available at this link.

As Lunar New Year festivities take place worldwide, there have been reports of avian influenza outbreaks in Asia and sporadic cases of human infections.
In light of this health risk, the World Health Organization (WHO) issued an updated Disease Outbreak News (DONs) on February 13, 2024, with prevention advice titled "Avian Influenza and Lunar New Year Festivities: Vigilance and Precautions" in February 2024.
During February 2024, WHO avian influenza alerts were issued for:
- Influenza A (H1N1) variant virus - Brazil, 7 February 2024
- Avian Influenza A (H5N1) - Cambodia, 8 February 2024
- Influenza A(H1N1) variant virus - Spain, 9 February 2024
- Avian Influenza A(H10N5) and Influenza A(H3N2) coinfection - China, 13 February
The WHO says vigilance remains crucial, although most human infections have been sporadic following contact with infected poultry and/or their environments, with no evidence of sustained human-to-human transmission.
Birds are the natural hosts for avian influenza viruses.
After an A(H5N1) virus outbreak in 1997 in poultry in Hong Kong SAR, China, since 2003, this avian and other influenza viruses have spread from Asia to Europe and Africa. I
Beginning in 2013, human infections with the influenza A(H7N9) virus were reported in China.
WHO DONs provide information on confirmed acute public health events or potential events of concern. For more details, please refer to the WHO Influenza (avian and other zoonotic) factsheet.
As of 2024, the U.S. government has invested tens of millions in vaccines protecting people from certain avian influenza viruses. Furthermore, the U.S. says annual flu shots are unlikely to protect people during avian influenza (bird flu) outbreaks.

As the global cholera epidemic enters another year, the World Health Organization (WHO) continues classifying cholera's resurgence as a grade 3 emergency, its highest internal level for emergencies.
And access to protective vaccines is decreasing.
On February 12, 2024, the WHO published its 11th multi-country cholera outbreak External Situation Report, which confirmed the global cholera response continues to be affected by a critical shortage of Oral Cholera Vaccines (OCV).
From January 2023 to January 2024, urgent requests for OCV surged, with 76 million OCV doses requested by 14 countries, while only 38 million doses were available during that period.
The global stockpile of cholera vaccines is awaiting replenishment, and all production up to March 8, 2024, will be allocated to approved requests.
The U.S. CDC recommends vaccination for people traveling to or living in areas of active cholera transmission. However, cholera vaccinations are not 100% effective.
Vaxchora, a single-dose, oral vaccine, is U.S. FDA-approved for use in people aged 2–64.
The WHO has approved three other OCVs.

In May 2022, when the mpox outbreak was first reported in Europe, most government health agencies started giving out Bavarian Nordic's JYNNEOS® (MVA-BN®, IMVAMUNE®) vaccine for free to eligible individuals.
As per a recent alert by the U.S. CDC COCA, only 25% of the two million eligible people have completed the JYNNEOS two-dose vaccination series in the U.S.
The CDC stated on February 12, 2024, that although the number of mpox outbreaks in the U.S. has decreased significantly since the summer of 2022, small clusters of the disease continue to occur, and severe manifestations of mpox, including deaths, are still being reported in 2024.
Furthermore, the CDC strongly urges clinicians, health departments, and community-based organizations to continue recommending the two-dose JYNNEOS vaccine to eligible individuals.
And the CDC is encouraging those who have only received one dose to get the second dose to obtain the best possible protection.
Moreover, the U.S. FDA recently approved JYNNEOS to prevent mpox infection, regardless of the mpox virus Clade.
This vaccine is generally available in major cities at health clinics and pharmacies. If you need more information about this update or wish to provide feedback, please get in touch with the CDC at [email protected].

Throughout the 2023-2024 respiratory syncytial virus (RSV) season, newly approved vaccines have been offered to pregnant women and older adults. As with all vaccines, it takes time to appreciate their ability to protect people from disease fully.
According to a report by TD Cowen's analyst Tyler Van Buren on February 8. 2024, Moderna Inc.'s mRNA-based RSV vaccine candidate may not be as effective as its competitors.
The Wall Street firm's report cites a Phase 3 clinical trial, which found that Moderna's mRNA-1345 vaccine candidate has an overall efficacy of 63.3% against two-symptom RSV disease after a follow-up of 8.6 months.
This is a significant change from a January 2023 reading, which showed mRNA-1345 had an efficacy of 84%.
"In the absence of head-to-head clinical trials, comparative conclusions regarding the safety and efficacy of mRNA-1345 relative to other RSV vaccines cannot be made," Moderna said in this abstract.
Moderna has previously confirmed it has submitted regulatory filings to the FDA for its RSV vaccine, indicating potential approvals ahead of the 2024-2025 RSV season in the U.S.
As of February 9, 2024, the U.S. CDC estimated the percentage of adults 60+ receiving an RSV vaccine was 22.4%. As of January 27, 2024, certain pregnant women's overall RSV vaccination rate was 16.2%.
As we approach Spring 2024, many individuals eagerly anticipate the end of the 2023-204 flu season. However, this flu season continues to pose a significant health risk for many people.
Globally, the World Health Organization recently released Influenza Update N° 464, indicating a decrease in influenza detections.
In North America, influenza activity is elevated, albeit declining.
The U.S. CDC reported on February 9, 2024, that seasonal influenza activity remained high across the nation, with certain regions, such as regions 5 and 7, experiencing significant increases.
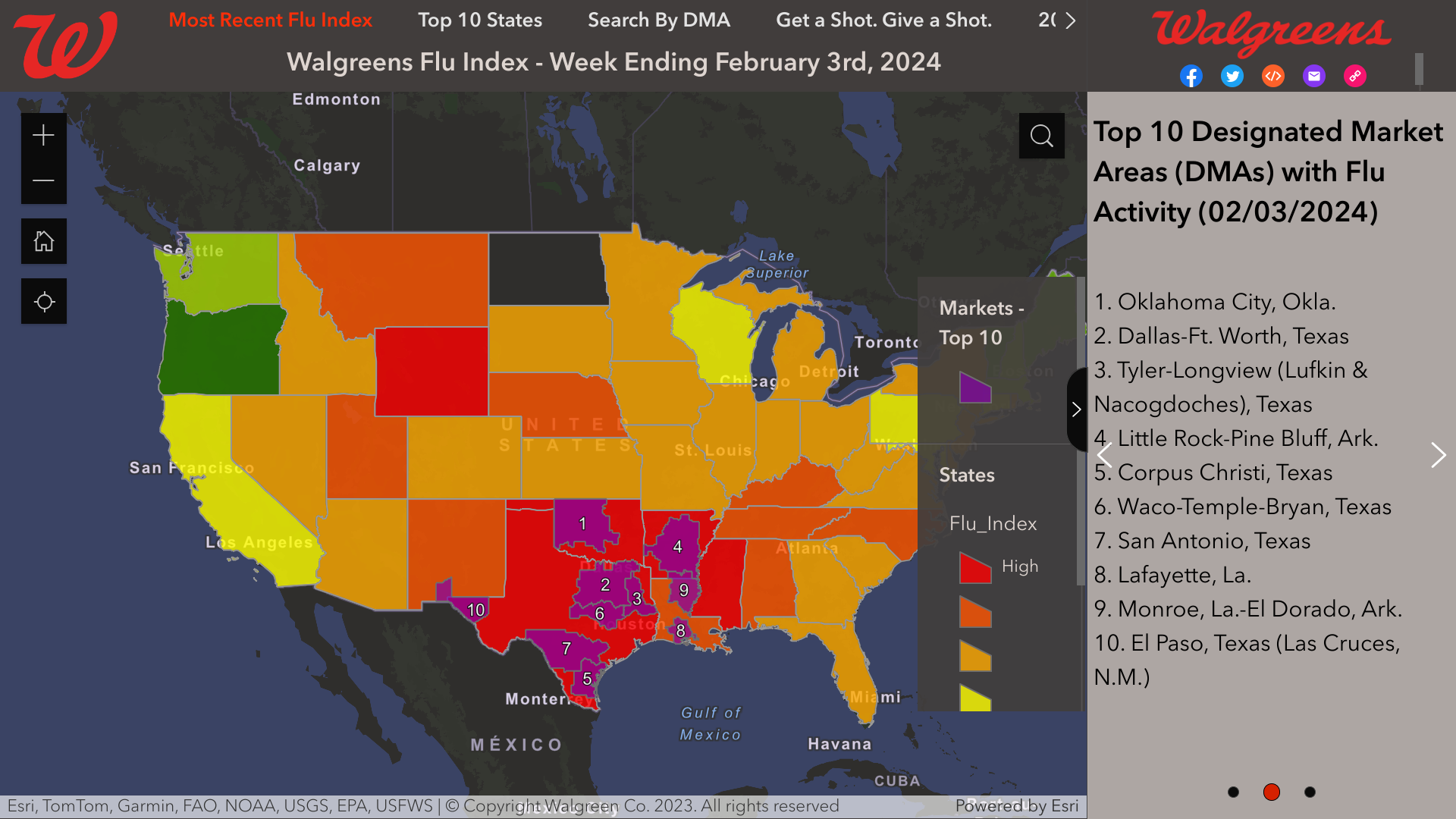
Additionally, the Walgreens Flu Index®, which provides a state and market-specific overview of flu activity, identified its top 10 cities confronting flu outbreaks as of February 3, 2024.
These cities are as follows:
- Oklahoma City
- Dallas-Ft. Worth
- Tyler-Longview
- Little Rock-Pine Bluff
- Corpus Christi
- Waco-Temple-Bryan
- San Antonio
- Lafayette
- Monroe, La.-El Dorado
- El Paso, Texas, including Las Cruces, N.M.
Note: Walgreens data is limited to the markets with its 8,700 pharmacy locations.
Unfortunately, the CDC confirmed eight influenza-associated pediatric deaths were reported during Week #5, bringing the 2023-2024 season total to 74 pediatric deaths.
The CDC continues recommending various flu shots for most people and encourages everyone to speak with a doctor, nurse, or pharmacist regarding any influenza questions.
As of late January 2024, over 157 million influenza vaccines were distributed in the U.S. this flu season.
The Maricopa County Department of Public Health (MCDPH) today confirmed a measles case involving an international visitor. This is the first measles case in 2024.
During 2021, there were 67 measles cases in Arizona.
Maricopa County, which includes the city of Phoenix, has a population of about 4.5 million.
On February 10, 2024, Dr. Nick Staab, assistant medical director for MCDPH, commented in a press release, "Measles is both highly infectious and completely preventable."
"We encourage residents to stay up-to-date on their vaccines and watch for symptoms of measles, especially if you are high-risk or unvaccinated," Dr. Staab added.
It can take up to 21 days after their last exposure for a person infected with measles to start showing symptoms.
Measles is a vaccine-preventable disease. Various measles vaccines are offered at most clinics and pharmacies in the U.S.
A CBS News investigation revealed on January 30, 2024, that at least 8,500 U.S. schools risk measles outbreaks in 2024 due to low vaccination rates. Data sources indicate about 90% of children in Arizona have been vaccinated against the measles virus.
During the first six weeks of 2024, measles cases were reported in Dayton, San Diego, Montgomery County, MD, Los Angeles, Philadelphia, Atlanta, Northern Virginia, Camden County, NJ, Kansas City, Wilmington, and Clark and Wahkiakum Washington counties.
As of January 25, 2024, the U.S. CDC reported a total of 9 measles cases were reported by four jurisdictions, mainly related to international travelers. During 2023, a total of 58 measles cases were reported by 20 jurisdictions.
In 2023, the CDC published a global Watch-Level 1, Travel Health Notice, identifying measles outbreaks in 47 countries.


