Search API
The Republic of South Africa's National Institute for Communicable Diseases (NICD) today reported the year-long measles outbreak continues in the northern provinces.
As of July 7, 2023, the NICD confirmed in the past week (week #25) that ten laboratory-confirmed measles cases were detected across the country, most of which were from Limpopo (6).
In week #25, Limpopo reported a total of 5 cases.
The vaccination campaign in Limpopo province targeting the 5-15 years age group has come to an end with overall vaccination coverage of 56%.
To achieve a higher coverage rate, the NICD is informed that a mop-up campaign will be conducted through the end of August 2023.
Various measles vaccines are available globally, and most health agencies recommend full vaccination before visiting measles outbreak areas.
The NICD says measles is a highly contagious disease caused by an airborne virus. Complications are more serious in those who catch measles as young infants and in children who are malnourished.
Infected patients with measles present with fever and with a rash. The rash looks like small, red, flat spots over the body. The rash does not form blisters, nor is it itchy or painful.
Other signs include cough, red eyes, and a running nose.
Complications of measles can include diarrhea, dehydration, brain infection, blindness, and death.

Novavax Inc. announced yesterday that it had reached an agreement with Canada, under which the country would pay $349.6 million to settle the forfeiting of certain doses of the company’s protein-based COVID-19 vaccine.
Novavax COVID-19 vaccine brands include Nuvaxovid™, CovoVax, NVX-CoV2373, and TAK-019.
Since authorization, over 100 million doses of Nuvaxovid have been distributed globally in about 40 markets.
As reported by BNN on July 7, 2023, this development results from a significant decrease in global demand for COVID-19 vaccines, leading to a surplus of unused doses.
The World Health Organization weekly epidemiological update edition #150 confirmed that during the previous 28 days, the COVID-19 pandemic has declined since mid-2022.
In addition to the settlement, Novavax also entered into a revised contract with Canada’s public works and government services department.
The terms of the advance purchase contract were amended to reflect the reduced number of vaccine doses due for delivery and the revised schedule for the remaining doses.
On June 6, 2023, the U.S. Food and Drug Administration confirmed Novavax COVID-19 Vaccine, Adjuvanted, was available in the U.S. for certain people. And on July 6, 2023, Nuvaxovid received Full Marketing Authorization in Europe as a primary series in individuals aged 12 and older and booster in adults.

The Florida Department of Health in Sarasota (DOH-Sarasota) recently reported two additional locally-acquired malaria infections caused by mosquito bites.
The Plasmodium species reported were Plasmodium vivax as of July 1, 2023.
According to Florida's surveillance report #26, the new malaria cases were in similar locations in greater Sarasota as the previous four cases with onsets in May and June 2023.
Regarding Travel-Associated Malaria Cases, twenty-three cases of malaria with onset in 2023 have been reported in Florida.
The countries of origin were Burundi, Côte D'Ivoire, Democratic Republic of the Congo (2), Equatorial Guinea, Ghana (2), Kenya, multiple countries (4), Nicaragua (2), Nigeria (2), Pakistan, Sierra Leone (2), Sudan, and Uganda (3).
The U.S. Centers for Disease Control and Prevention says malaria is a mosquito-borne disease.
Left untreated, malaria infections can develop severe complications. In 2020, an estimated 241 million cases of malaria occurred worldwide.
Malaria vaccines are currently in use in Africa and are reported to be effective at preventing disease.
As of July 7, 2023, the U.S. Food and Drug Administration had not approved a malaria vaccine for use in Florida.
Novavax, Inc. announced yesterday that its protein-based COVID-19 vaccine had been granted full Marketing Authorization (MA) by the European Commission in the European Union (EU).
The Nuvaxovid™ (NVX-CoV2373) vaccine is now fully authorized for use in the EU as a primary series in individuals aged 12 and older.
And as a booster dose in adults aged 18 and older to prevent COVID-19.
During the diminishing pandemic, Novavax's COVID vaccine has been authorized for use in more than 40 markets worldwide.
"This Marketing Authorization establishes the foundation for all future regulatory approvals for updated versions of our COVID vaccine, a necessity to ensure we can quickly get our vaccine to individuals in the EU," said John C. Jacobs, President and Chief Executive Officer, Novavax, in a press release on July 6, 2023.
"In addition to the EU, we are preparing to file for full approval in the U.S. and other markets and are committed to ensuring protein-based options are available worldwide."
"Vaccine choice remains an integral part of public health measures."
The U.S. Food and Drug Administration has not yet approved the trade name Nuvaxovid™, nor has it been approved or licensed.
However, as of July 7, 2023, it has been authorized for emergency use under an Emergency Use Authorization for various people. In Europe, over ten COVID-19 vaccines are available.

Eisai Co., Ltd. and Biogen Inc. announced today that the U.S. Food and Drug Administration (FDA) had approved the supplemental Biologics License Application (sBLA) supporting the traditional approval of LEQEMBI® (lecanemab-irmb) 100 mg/mL injection for intravenous use.
This approval makes LEQEMBI the first and only approved treatment shown to reduce the rate of disease progression and slow cognitive and functional decline in adults with Alzheimer's disease (AD).
In a phase 3 clinical trial, LEQEMBI demonstrated clinically meaningful slowing of cognitive and functional decline in a patient group generalizable to U.S. Medicare beneficiaries, which included a mix of racial and ethnic groups, patients with common comorbid conditions, concomitant medications, and patients with mild cognitive impairment (MCI) due to AD or mild AD.
Treatment with LEQEMBI should be initiated in patients with MCI or mild dementia stage of disease, the population in which treatment was initiated in clinical trials.
Furthermore, CMS confirmed that broader coverage of LEQEMBI is now available. The CMS-facilitated registry is now available for healthcare professionals to submit required patient data to CMS.
This action will facilitate reimbursement for and access to LEQEMBI across a broad range of healthcare settings in the U.S.
As of July 7, 2023, there are no approved Alzheimer's disease vaccines in the U.S.

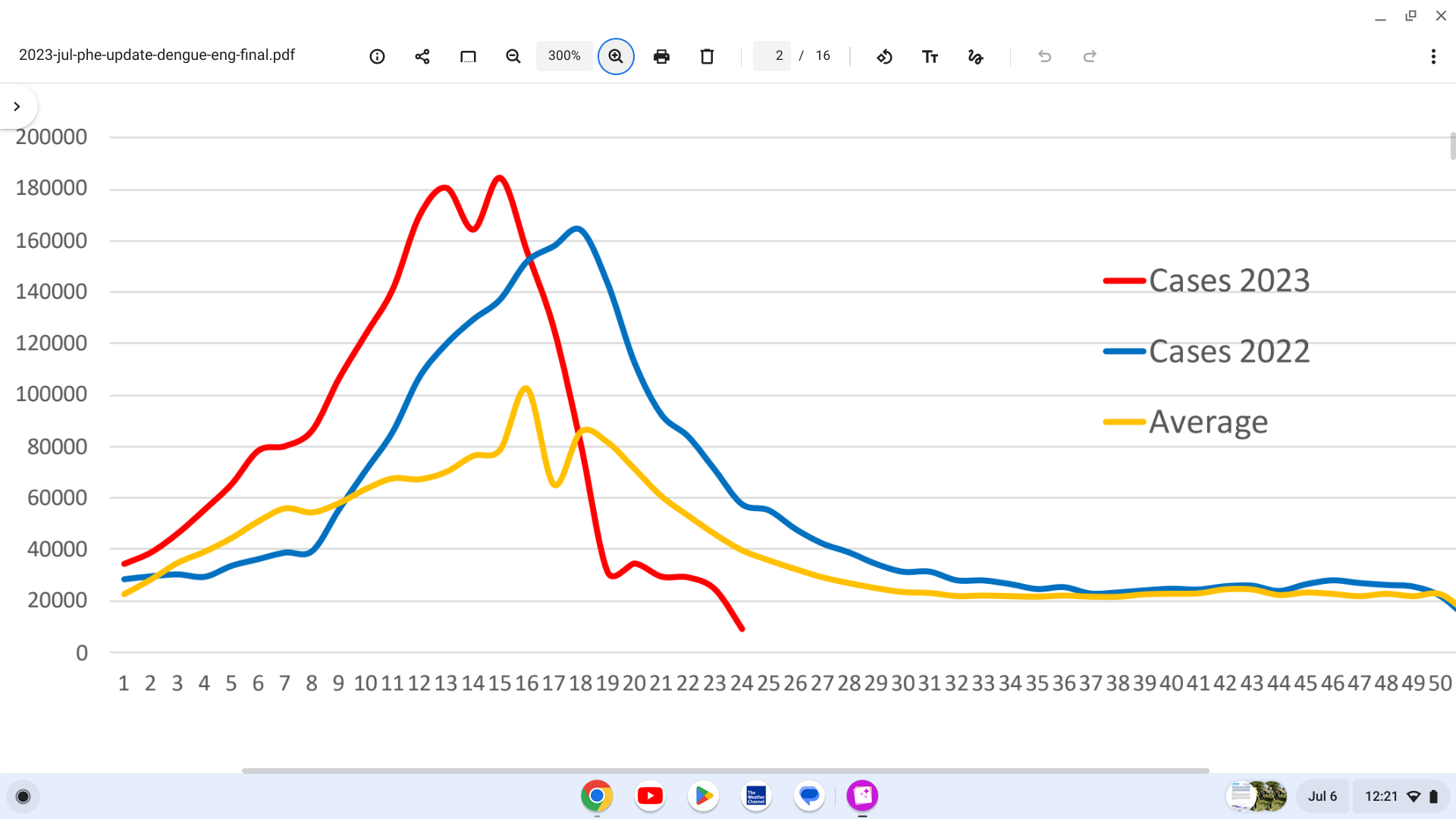
The Pan American Health Organization / World Health Organization (PAHO/WHO) recently stated the seasonality of dengue in Central America and the Caribbean is a concern and recommends that Member States prepare plans to face possible outbreaks during the summer of 2023.
In 2023, through week #24, of the 2,102,848 dengue cases reported in the Region, 3,201 (0.15%) were classified as severe dengue.
Additionally, in the same period, 876 deaths were reported in the Region (case fatality rate: 0.042%).
The greatest number of dengue cases was observed in Brazil, with 1,515,460 cases, followed by Peru, with 169,504
patients and Bolivia with 133,452 cases.
Regarding the number of severe dengue cases, Brazil led with 654 instances, Colombia with 652 instances, Peru with 597, Bolivia with 590 patients, and Mexico with 573 cases, says the PAHO/WHO.
According to the Centers for Disease Control and Prevention (CDC), dengue is a vectorborne infectious disease caused by four dengue viruses and is endemic in about 125 countries.
The CDC recently reissued a Travel Health Advisory regarding dengue outbreaks in Asia and the Pacific Islands.
In the U.S., Florida has reported the most locally-acquire and travel-related dengue cases in 2023.
Dengue is also a vaccine-preventable disease. In 2022, a seconded dengue vaccine was approved for use by certain countries.
The Lancet Infectious Diseases recently published results from a phase 1 clinical trial of a Lyme disease vaccine candidate, showing that Valneva's VLA15 produces a strong but waning immune response against six common strains of the Borrelia burgdorferi bacterium found in Europe and the United States.
Valneva Austria researchers led this company-sponsored, partially randomized, observer-masked study of the novel, multivalent outer surface protein A (OspA) subunit vaccine candidate.
OspA is one of the most dominant surface proteins expressed by the bacteria when present in a tick.
VLA15 produced immune responses for all strains, but responses were greater in the higher-dose adjuvanted groups. And one month after the third dose, responses declined, reaching baseline by one year.
And a booster dose given 13 months after the first dose triggered a strong immune response for about six months.
This study's findings are good news since Lyme borreliosis is the most common tick-borne disease in the northern hemisphere.
In Europe, there are estimated to be more than 200,000 cases each year. And in the U.S., approximately 30,000 patients per year.
In a related commentary also published by The Lancet, Nicole Bézay, and colleagues reported Valneva's novel vaccine represents a milestone in our fight against Lyme disease."
Initially developed by France-based Valneva SE, New York-based Pfizer, Inc. is VLA15's current development and commercialization collaboration.
Pfizer previously indicated it could submit a Biologics License Application in 2025 and Marketing Authorization Application in Europe in 2026, subject to positive data.

The World Health Organization (WHO) today announced that 12 African countries will receive about 18 million doses of an approved malaria vaccine over the next two years.
On July 5, 2023, the WHO confirmed GSK's Mosquirix™ RTS,S/AS01 malaria vaccine will continue in Ghana, Kenya, and Malawi.
The first vaccine doses are expected to arrive in countries during the last quarter of 2023, with countries starting to roll them out by early 2024.
The Mosquirix vaccine has been administered to over 1.7 million children since 2019. It has been shown to be safe and effective, resulting in a substantial reduction in severe malaria and a fall in child deaths.
Mosquirix allocations were also made for new vaccine introductions in Benin, Burkina Faso, Burundi, Cameroon, the Democratic Republic of the Congo, Liberia, Niger, Sierra Leone, and Uganda.
These allocations were determined by applying the principles outlined in the Framework for allocating limited malaria vaccine supply.
Malaria remains one of Africa's deadliest diseases, killing nearly half a million children under five and accounting for approximately 95% of global malaria cases in 2021.
A second malaria vaccine, R21/Matrix-M™, developed by Oxford University and manufactured by the Serum Institute of India, could also be prequalified by WHO soon.
As of July 5, 2023, these malaria vaccines are not offered in the U.S. (Florida) or Costa Rica.


