Search API
A systematic review and meta-analysis published today in Antimicrobial Resistance and Infection Control journal show that influenza vaccination is associated with significantly reduced antibiotic use.
The study focused on data from randomized controlled trials (RCT) and observational studies.
The RCTs showed that the effect of influenza vaccination on the number of antibiotic prescriptions or days of antibiotic use (Ratio of Means (RoM) 0.71, 95% CI 0.62–0.83) is stronger compared to the effect of pneumococcal vaccination (RoM 0.92, 95% CI 0.85–1.00).
These studies also confirm a reduction in the proportion of people receiving antibiotics after influenza vaccination (Risk Ratio (RR) 0.63, 95% CI 0.51–0.79).
And the effect of influenza vaccination in the European and American regions ranged from RoM 0.63 and 0.87 to RR 0.70 and 0.66, respectively.
However, the evidence from observational studies supports these findings but presents a less consistent picture.
Announced on July 14, 2023, this data supported the use of influenza vaccination as an important public health intervention to reduce antibiotic use and possibly control antimicrobial resistance.
In the northern hemisphere, the 2023-2024 flu season is forecasted to begin in the fall, with an ample supply of influenza vaccines available at most clinics and pharmacies in the U.S.
Gilead Sciences, Inc. today announced that the U.S. Food and Drug Administration (FDA) approved a supplemental new drug application (sNDA) for the use of Veklury® (remdesivir) in COVID-19 patients with severe renal impairment, including those on dialysis.
With this approval, Veklury is now the first and only approved antiviral COVID-19 treatment that can be used across all stages of renal disease.
This is essential news since more than 37 million people in the U.S. are estimated to have chronic kidney disease (CKD) and are at increased risk of COVID-19-related morbidity and mortality.
The FDA approval follows the European Commission's decision to extend the approved use of Veklury on June 26, 2023.
"Patients with advanced CKD and end-stage kidney disease (ESKD) are at high risk for severe COVID-19 with hospitalization and mortality rates remaining high, even for those who are vaccinated. With limited clinical trial information for COVID-19 patients with advanced CKD and ESKD, few antiviral treatment options currently exist for this population," said Meghan Sise, MD, Department of Nephrology at Massachusetts General Hospital, in a press release on July 14, 2023.
"This latest update to the prescribing information for remdesivir now includes patients with advanced CKD and ESKD, and this is an important advance for a population that remains highly vulnerable to the impacts of COVID-19."
The updated prescribing information for Veklury announced today does not require dose adjustments for renal-impaired patients and removes the requirement for eGFR testing before or during treatment with Veklury.
The leading cause of mosquito-borne disease in the continental United States is spread to people by the bite of an infected mosquito and has recently begun to spread in 2023.
Fortunately, most people infected with West Nile virus (WNV) do not feel sick, says the Centers for Disease Control and Prevention (CDC).
However, about 1 out of 150 infected people develop a serious, sometimes fatal, illness.
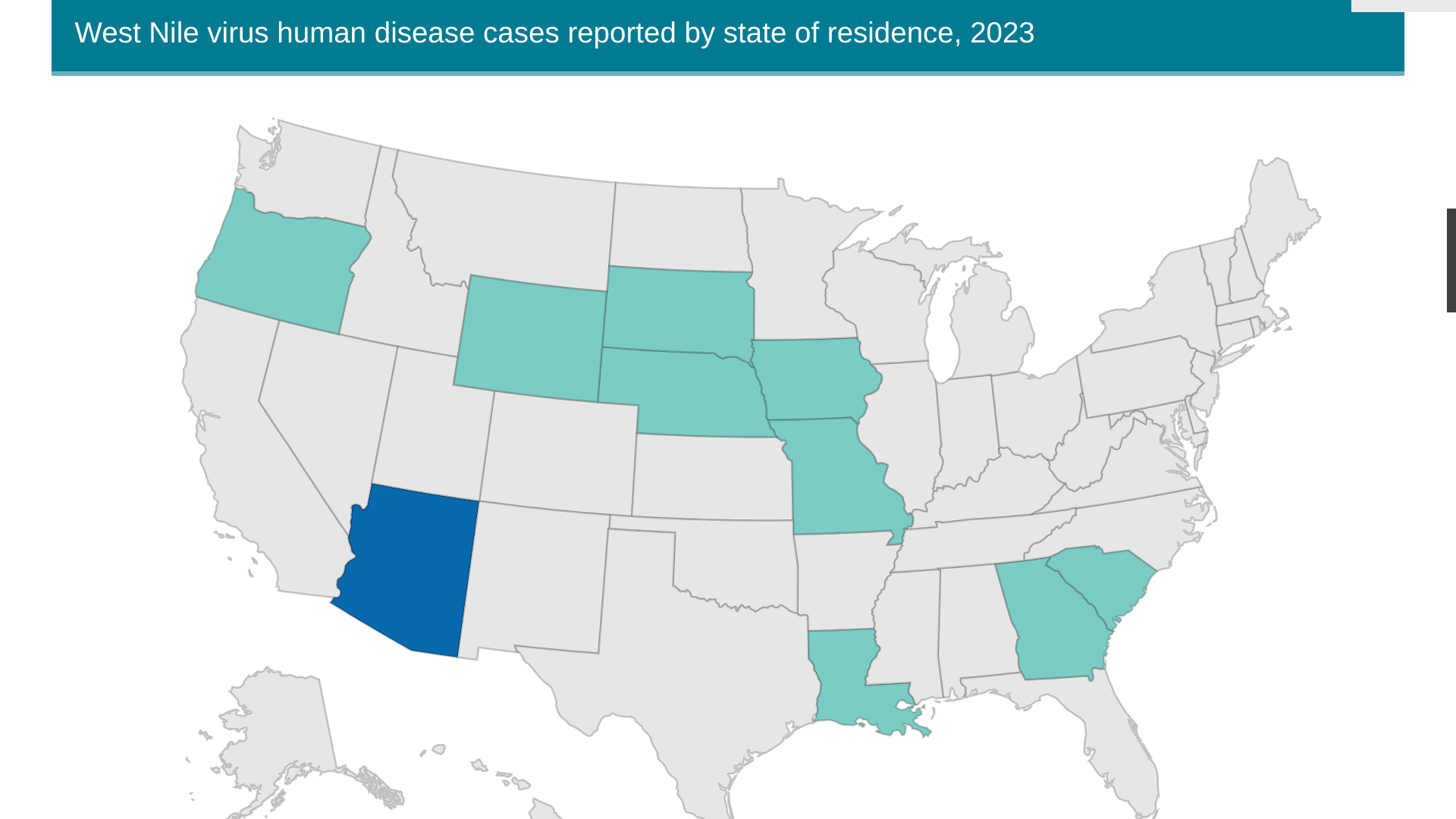
As of July 11, 2023, the CDC reported ten states have reported 36 human cases of WNV this year. So far, 23 neuroinvasive infections have been reported.
Not reported by the CDC was a WNV case confirmed in Dallas, Texas, during July 2023.
Arizona has the most WNV cases, with 25 reported in 2023. In 2021, Arizona reported over 1,700 WNV cases.
In 2022, there were 1,126 WNV cases, including 90 deaths in the U.S.
"It's important for people to be aware that there are many diseases transmitted by mosquitoes found in Texas," said Texas Department of State Health Services Commissioner Jennifer Shuford, MD, MPH, in a related press release.
"Most of these (WNV) diseases cause mild illness, but in rare instances, diseases like dengue or Zika can cause severe illness."
"We've even had a locally acquired malaria case in Texas this year, which underscores the importance of taking precautions to prevent mosquito bites."
There are no vaccines to prevent or medications to treat WNV in people as of July 14, 2023.
Genentech today announced that the Phase III OCARINA II clinical trial evaluating Ocrevus® (ocrelizumab) as a twice-a-year 10-minute subcutaneous injection met its primary and secondary endpoints in patients with relapsing forms of Multiple Sclerosis (MS) or primary progressive MS (RMS or PPMS).
In this clinical trial, Ocrevus subcutaneous injection was shown to be non-inferior to Ocrevus given by intravenous infusion (IV), as measured by pharmacokinetics (levels in the blood) over 12 weeks.
Additionally, Ocrevus subcutaneous injection was comparable with Ocrevus IV in controlling brain magnetic resonance imaging lesion activity over 12 weeks.
“These results give people living with MS the possibility to receive the transformational benefits of Ocrevus in the way best suited to their lives while freeing up time and healthcare resources,” said Levi Garraway, M.D., Ph.D., Genentech’s chief medical officer and head of Global Product Development, in a press release on July 13, 2023.
“This new subcutaneous injection will allow Ocrevus to be administered in 10 minutes twice a year, helping people living with MS to spend less time in treatment for this disease.’’
Ocrevus is not a vaccine but is a humanized monoclonal antibody designed to target CD20-positive B cells, a specific type of immune cell thought to be a key contributor to nerve cell insulation and support and nerve cell damage.
The Ocrevus 10-minute injection is designed to be administered without the need for IV infrastructure. Hence, it can potentially expand the usage of Ocrevus in MS centers without IV infrastructure or those with IV capacity limitations.
It also retains the twice-yearly dosing regimen of Ocrevus IV that has shown high persistence and adherence since becoming a standard of care MS treatment.
This provides an additional delivery option so that the administration of Ocrevus can be matched to the individual needs of patients and healthcare professionals.
The investigational subcutaneous formulation combines Ocrevus with Halozyme Therapeutics’ Enhanze® drug delivery technology.
Ocrevus remains the first and only therapy approved for RMS and PPMS, and more than 300,000 people have been treated globally.

The current outbreaks of avian influenza ("bird flu") continue to cause devastation in animal populations worldwide. Although largely affecting animals, these outbreaks pose ongoing risks to humans, says the World Health Organization (WHO).
The WHO wrote today that an increasing number of H5N1 avian influenza detections among mammals, which are biologically closer to humans than birds, raises concern that the virus might adapt to infect humans more easily.
In addition, some mammals may act as mixing vessels for influenza viruses, leading to the emergence of new viruses that could be more harmful to animals and humans.
Announced on July 12, 2023, the WHO, Food and Agriculture Organization of the United Nations, and the World Organisation for Animal Health urge countries to work together across sectors to save as many animals as possible and protect people.
Sporadic influenza A(H5N1) clade 2.3.4.4b virus detections in humans have been reported but remain very rare, with 8 cases reported since December 2021.
But only one case in the U.S.
Infections in humans can cause severe disease with a high mortality rate, says the WHO.
The human cases detected thus far are mostly linked to close contact with infected birds and contaminated environments.
"With the information available so far, the virus does not appear to be able to transmit from one person to another easily, but vigilance is needed to identify any evolution in the virus that can change that," said Dr. Sylvie Briand, Director of Epidemic and Pandemic Preparedness and Prevention, WHO, in a related press release.
"We encourage all countries to increase their ability to monitor these viruses and to detect any human cases. This is especially important as the virus now affects countries with limited prior experience in avian flu surveillance."
Studies are underway to identify any changes in the bird flu virus that may help the virus to spread more easily among mammals, including humans, says the WHO.
The U.S. CDC's Situation Summary issued as of July 5, 2023, confirmed the current risk to the public from bird flu viruses remains low. However, continued sporadic human infections are anticipated.
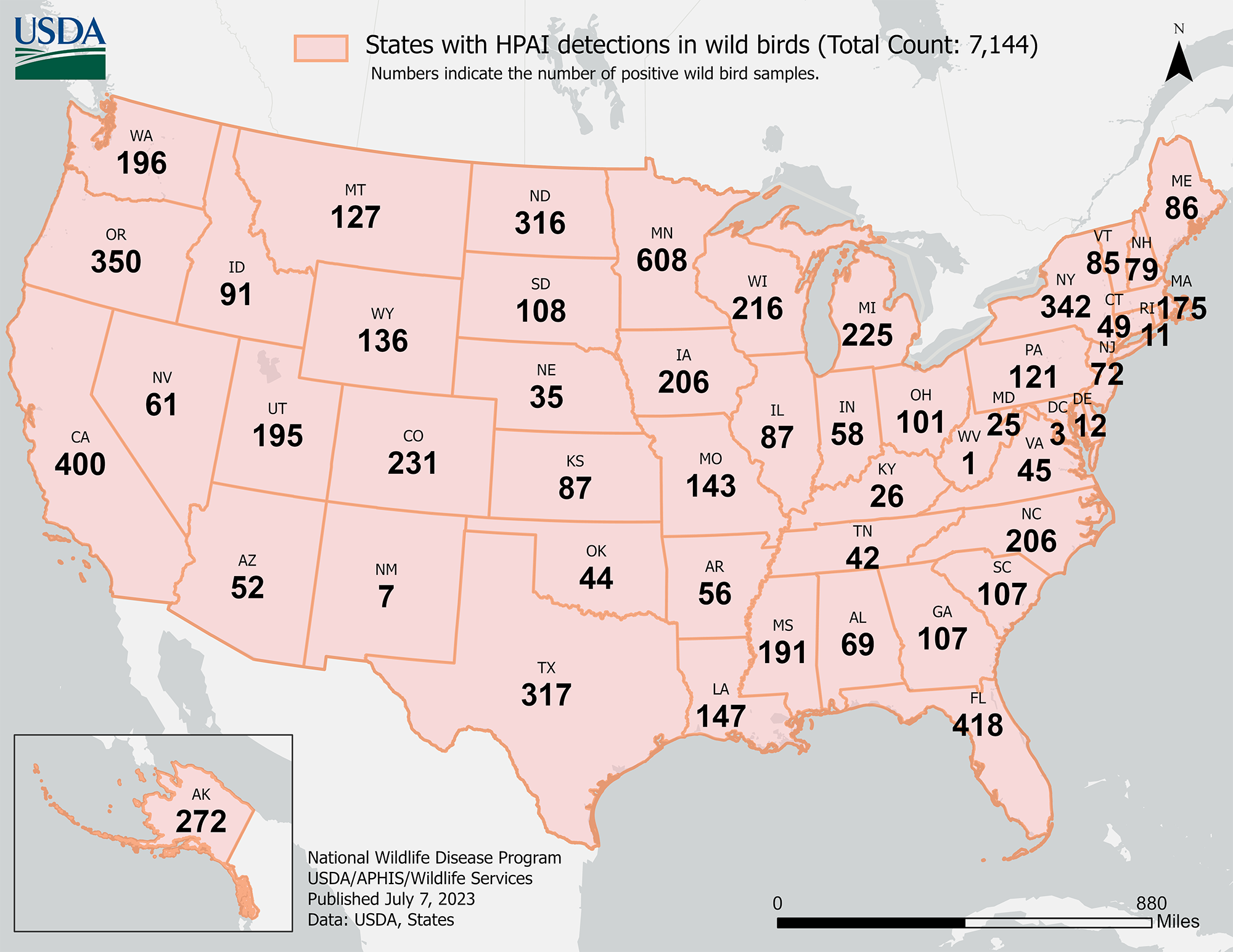
During this bird flu outbreak, there have been 7,105 virus detections in 47 U.S. states.
As of July 12, 2023, the U.S. government has stockpiled and approved avian influenza vaccines.
GSK plc recently announced that the Medicines and Healthcare products Regulatory Agency (MHRA) has authorized Arexvy for active immunization for the prevention of lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) in adults 60 years of age and older.
This is the first time an RSV vaccine for older adults has been authorized in Great Britain.
Neale Belson, GSK's Senior Vice President and General Manager UK, said in a press release on July 10, 2023, "Our ambition is to help protect adults 60 years of age and older in the U.K. who are at risk from RSV disease, including those with underlying medical conditions, who drive the majority of RSV hospitalizations."
"This authorization for Arexvy means eligible adults can be vaccinated against RSV disease for the first time, reinforcing GSK's long history of vaccine innovation."
RSV is a common, contagious respiratory virus that leads to an estimated 175,000 GP visits, 14,000 hospitalizations, and 8,000 deaths yearly in adults aged 60 and over in the U.K.
Recent studies indicate that the burden of RSV disease may be even greater than that of influenza in hospitalized older adults.
In the U.S., two RSV vaccines have been approved, and several RSV vaccine candidates are conducting late-stage clinical trials.
Precision Vaccinations publishes RSV seasonal trends for 2023.

The future vaccination plans for U.S. travelers and those living in dengue-outbreak areas, such as Puerto Rico, were disrupted yesterday.
Takeda announced on July 11, 2023, that the Company had voluntarily withdrawn the U.S. Biologics License Application (BLA) for its dengue vaccine candidate, TAK-003, following discussions with the U.S. Food and Drug Administration (FDA).
Takeda's press release stated that aspects of data collection could not be addressed within the current BLA review cycle.
On November 22, 2022, Takeda announced that the FDA had accepted and granted priority review of the TAK-003 BLA.
TAK-003, known internationally as QDENGA®, is approved in multiple endemic and non-endemic countries, such as the United Kingdom, Europe, and Brazil.
Gary Dubin, M.D., president of Takeda's Vaccines Business Unit, commented in a related press release, "The urgent global need to combat the growing burden of dengue remains, and we will continue to progress regulatory reviews and provide access for people living in and traveling to dengue-endemic areas while we work to determine next steps in the U.S."
QDENGA® is a tetravalent dengue vaccine preventing Dengue Fever or Severe Dengue caused by any of the four serotypes of the dengue virus.
During 2023, locally-acquired dengue has been confirmed in Florida and Texas.
While other second-generation dengue vaccine candidates are in development, the initial FDA-approved dengue vaccine Dengvaxia® remains available in the U.S. but has specific pre-vaccination requirements.