Search API
Following detecting a new emergence of variant poliovirus type 2 in six wastewater samples in the Port Sudan locality, Red Sea State, the Republic of Sudan recently announced polio vaccinations will begin in April 2024.
Sudan's Federal Ministry of Health (FMOH) announced on March 11, 2024, that this new detection comes 14 months after Sudan declared an outbreak of variant poliovirus type 2 from an emergence of the virus.
While no vaccination campaign has occurred since April 2023, surveillance for poliovirus in children focused on the most common indicator of a polio infection, acute flaccid paralysis.
While no child has been paralyzed due to the new emergence, detecting poliovirus in wastewater samples puts children across the country at high risk.
"The new detection has only redoubled our commitment to safeguarding our children's future. In collaboration with partners, we are mobilizing an outbreak response campaign to ensure that every child under five years in inaccessible areas receives the polio vaccine, and special plans will follow for hard-to-reach areas," said Dr. Dalya Eltayeb, Director-General of Primary Health Care in Sudan's FMOH, in a media statement.
In October 2022, the FMOH distributed 10.3 million doses of oral polio vaccine (OPV) in Sudan.
This year, Sudan is deploying the WHO-authorized type 2 novel oral polio (nOPV2) vaccine, which has been used 1 billion times by over 30 countries.
nOPV2 is reported to be more genetically stable than OPVs, with a lower risk of reversion to neurovirulence and less likely to mutate and cause paralysis.
While the nOPV2 vaccine is not approved in the United States, the government's vaccine committee considered its use under specific conditions during its February 28, 2024 meeting.
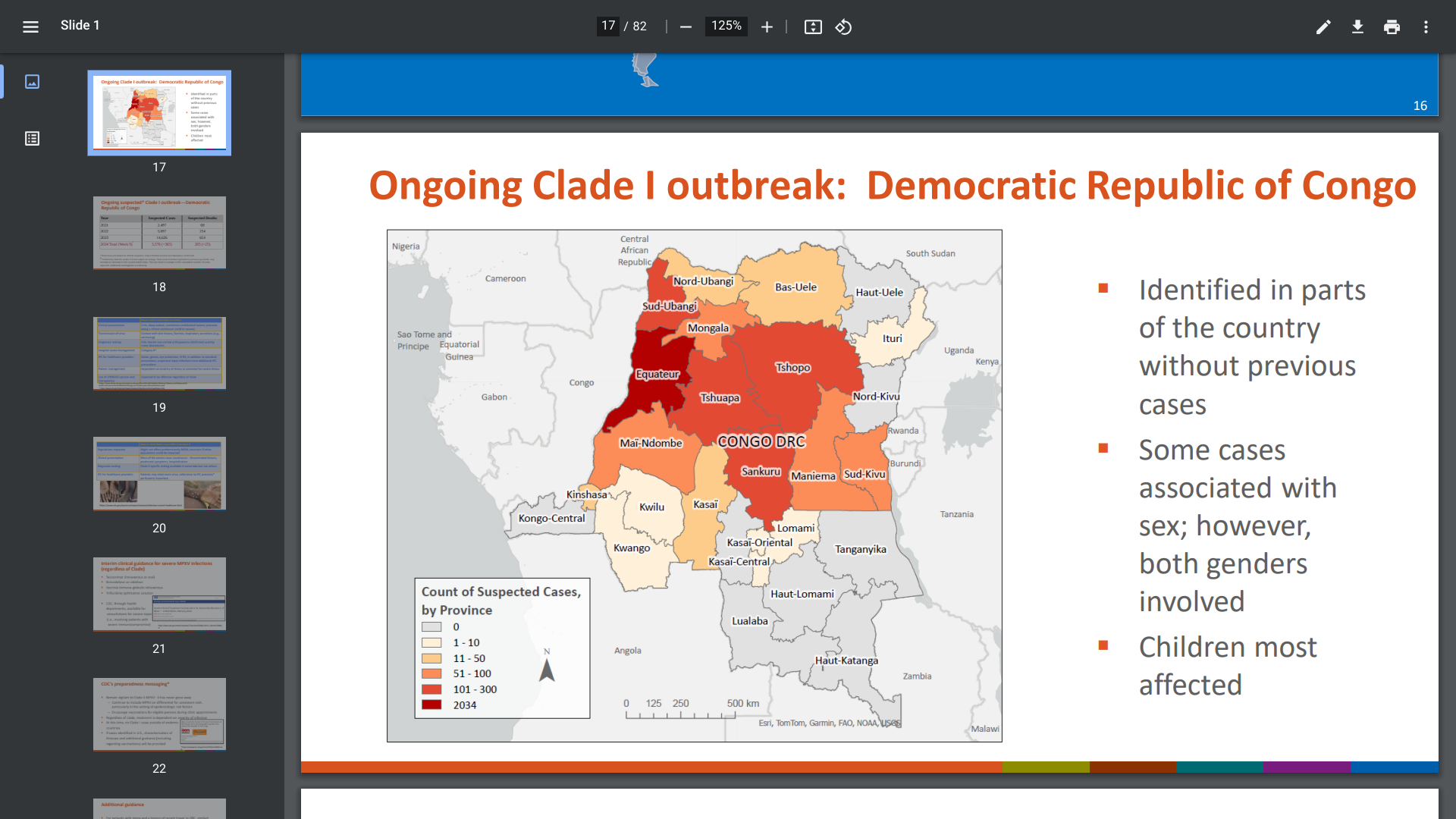
Various reports suggest that Africa's mpox Clade 1 outbreak has recently intensified. As of mid-March, the Democratic Republic of the Congo (DRC), Eurosurveillance, and the World Health Organization released updated mpox outbreak figures for 2024.
These reports revealed 14,626 suspected cases for the last year, with 654 fatalities. This equates to a case-fatality rate (CFR) of 4.5%.
Separately, the U.S. Centers for Disease Control and Prevention (CDC) and the Infectious Diseases Society of America estimated the CFR to be approximately 7.4% in the DRC.
Despite this concerning situation, limited genomic information is available on the circulating mpox viruses, which suggests that they belong to Clade I.
This finding demonstrates that mpox transmission through sexual contact may extend beyond clade IIb, says the CDC.
During a digital briefing on March 14, 2024, Agam Rao, MD CAPT, U.S. Public Health Service, reconfirmed the Advisory Committee on Immunization Practices recommended on October 25, 2023, vaccination with the 2-dose JYNNEOS® (MVA-BN®, IMVAMUNE®) vaccine series for persons aged 18 years and older at risk for mpox.
Since the Clade 2 outbreak began in May 2022, the overall vaccine coverage in the U.S. for one dose is 40%, and for two doses, it is 25%.
The U.S. Food and Drug Administration initially approved JYNNEOS for smallpox in 2019. JYNNEOS remains available in the U.S. at specific clinics and community pharmacies.
With an estimated 11 to 20 million typhoid fever cases every year and about 120,000 related deaths, global health leaders are aggressively improving access to new vaccines.
The World Health Organization (WHO) recently confirmed its recommendation to use three vaccines to control endemic and epidemic typhoid fever.
In late February 2024, SK bioscience and the International Vaccine Institute (IVI) announced that the SKYTyphoid™ typhoid conjugate vaccine had received prequalification (PQ) from the World Health Organization.
WHO PQ certifies a vaccine's safety, efficacy, and GMP by evaluating its manufacturing process, quality, and clinical trial results according to stringent standards.
SKYTyphoid utilizes the 'purified Vi polysaccharide-diphtheria toxoid conjugate' method, which conjugates diphtheria toxin protein (diphtheria toxoid), which acts as a carrier, to polysaccharide of typhoid bacteria, which acts as an antigen.
Adopting conjugation technology, the vaccine is safe for infants and young children aged six months to 2 years. It is expected to provide sufficient immune response and long-term protection with a single dose compared to existing oral live or polysaccharide typhoid vaccines.
SKYTyphoid initially obtained a licensure in Korea in 2022.
Dr. Sushant Sahastrabuddhe, Director of IVI's Typhoid program, said in a February 23, 204 press release, "The WHO licensure of SKYTyphoid... will diversify and expand the supply of TVCs and help improve vaccine access in the endemic countries. With SK's commitment to making the vaccine for global public health at a competitive price, SKYTyphoid will play an important role in typhoid prevention globally."
SK bioscience plans to start supplying the vaccine as soon as possible and expand global supply through public procurement markets including typhoid endemic countries.
Typhoid fever is transmitted by consuming raw or undercooked food or water contaminated with the feces of an infected person.
In 2024, there are significant typhoid fever outbreaks in sub-Saharan Africa.
In March 2024, local media reported that Taiwan confirmed its first locally acquired typhoid fever case this year. Since 2019, Taiwan has accumulated 49 typhoid cases, 18 of which were domestic cases.
In the United States, about 5,700 people get typhoid fever each year, and 620 of those people are hospitalized.
There are currently two typhoid fever vaccines available in the United States.

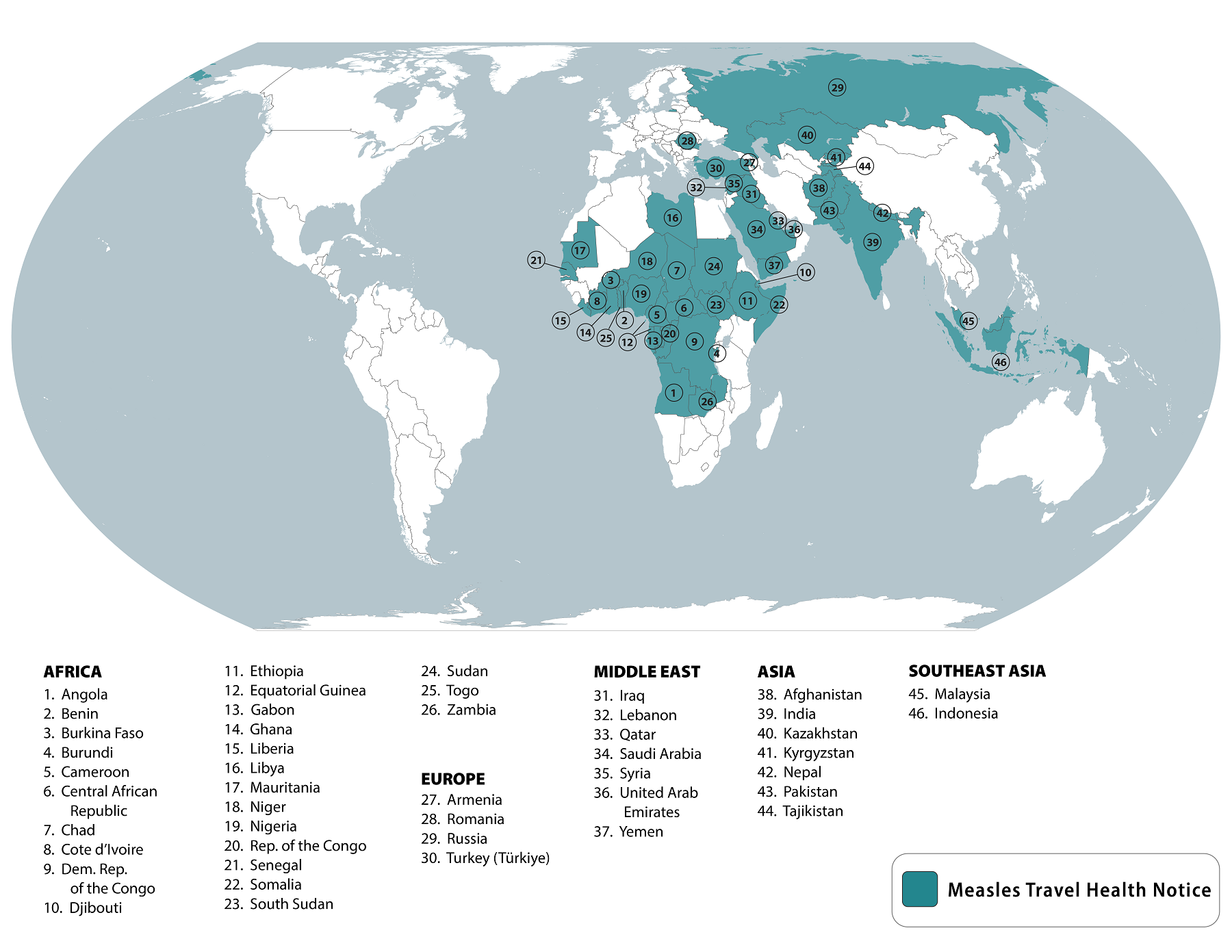
The United States government recently issued a strong warning regarding the ongoing measles outbreaks in 46 countries.
And the federal government is supporting Chicago's efforts to curb the spread of the highly infectious measles virus.
To clarify its vaccination recommendations, on March 13, 2024, the U.S. Centers for Disease Control and Prevention (CDC) updated its Level 1 Travel Health Advisory to ensure that all international travelers are fully protected against this vaccine-preventable disease.
Vaccination with a measles-containing vaccine is the best way to make sure that you are protected. Even young infants should receive one dose of the MMR vaccine, says the CDC.
If you are unsure if you or your travel companions are fully protected against measles, schedule an appointment to see your clinician or pharmacist at least six weeks before traveling so that you have enough time to get vaccinated.
However, some people should not get a measles-containing vaccine or should wait. If you don't think you can safely receive one, talk to your clinician and consider making alternative travel plans.
Individuals must take the necessary steps to safeguard their health and the health of others by following these guidelines, says the CDC.
As of March 16, 2024, measles vaccines are offered at community pharmacies in the U.S.
An early release study published in The Lancet Global Health (April 2024) has revealed that sub-Saharan African countries are experiencing a significant burden of typhoid fever.
Combined with the threat of typhoid strains resistant to antibiotic treatment, this necessitates more robust prevention strategies.
These researchers wrote that such strategies should include using and implementing typhoid conjugate vaccines (TCVs) in endemic settings and improving access to safe water, sanitation, and hygiene.
Currently, the World Health Organization (WHO) has prequalified two TCVs that are effective with children.
In February 2024, IVI and SK bioscience announced that a third TCV, SKYTyphoid™, also achieved a WHO qualification, paving the way for public procurement and increasing the global supply.
Dr. Birkneh Tilahun Tadesse, Associate Director General at IVI and Head of the Real-World Evidence Department, explained in a press release on March 12, 2024, “Through these vaccine effectiveness studies, we aim to show the full public health value of TCV in settings that are directly impacted by a high burden of typhoid fever.”
He adds, “Our final objective, of course, is to eliminate typhoid or to at least reduce the burden to low incidence levels, and that’s what we are attempting in Fiji with an island-wide vaccination campaign.”
There are about 16 million typhoid cases every year, with 140,000 deaths.
However, with generic symptoms such as fever, fatigue, and abdominal pain and the need for blood culture sampling to make a definitive diagnosis, it is difficult for governments to capture the actual burden of typhoid in their countries.
Results from a phase 3 randomized controlled clinical trial published in The Lancet found one dose of the conjugate typhoid vaccine had an estimated efficacy of 78.3% in children ages nine months to 12 years and remained strong over four years.
The U.S. CDC says that in March 2024, vaccination is recommended for people traveling to places where typhoid fever is common. International travelers should visit a healthcare provider or travel vaccine pharmacy to discuss prevention options.
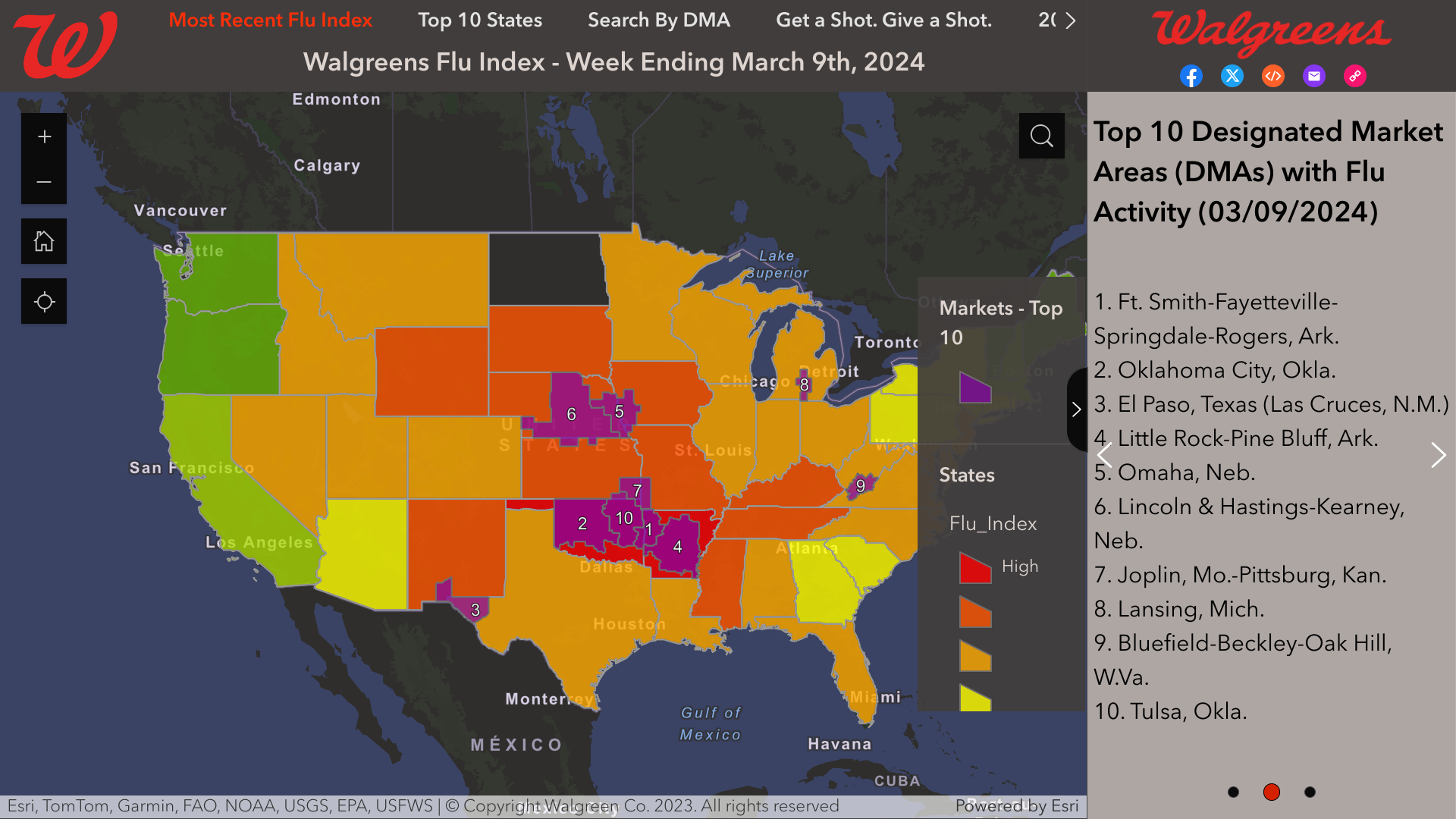
The Walgreens Flu Index© offers the latest information on flu activity specific to individual cities in the U.S.
This information is compiled using retail prescription data for antiviral medications used to treat influenza across all Walgreens locations. It offers insight into which populations are experiencing the highest incidence of the flu.
As of March 9, 2024, the Index listed these cities:
- Ft. Smith-Fayetteville-Springdale-Rogers, Ark.
- Oklahoma City, Okla.
- El Paso, Texas (Las Cruces, N.M.)
- Little Rock-Pine Bluff, Ark.
- Omaha, Neb.
- Lincoln & Hastings-Kearney, Neb.
- Joplin, Mo.-Pittsburg, Kan.
- Lansing, Mich.
- Bluefield-Beckley-Oak Hill, W.Va.
- Tulsa, Okla.
It's important to note that the Flu Index is not intended to show the severity or intensity of flu activity.
From a severity perspective, the U.S. CDC reported on March 15, 2024, that thirteen influenza-associated pediatric deaths occurring during the 2023-2024 season were reported last week throughout the United States, bringing the season total to 116 pediatric deaths.
The CDC encourages everyone to discuss flu vaccines or treatments with a healthcare provider.
Calder Biosciences, Inc. announced today that the journal Nature Communications published an article that debuts and validates the application of Calder's '3D Vaxlock' platform technology.
When applied to the Respiratory Syncytial Virus (RSV) F protein as a vaccine immunogen, Calder's 3D Vaxlock technology achieves an unprecedented 11X more potent immune response than the standard industry comparator.
The 11-fold higher responses are measured in terms of antibodies generated that neutralize the virus on contact, thus preventing infection.
The technology's application shows that the prefusion conformation of RSV fusion protein can be stabilized with two engineered dityrosine crosslinks (DT-preF), markedly improving its stability and shelf-life.
Calder's vaccine also demonstrates improvements in the quality of elicited immune responses since a greater proportion of the antibodies neutralize the virus.
"There remains an urgent need for vaccines that provide good protection for 75+ older adults and the frail. Protecting newborn children for a longer period through maternal vaccination also remains an important goal," said Florian Schödel, MD, a Calder's Scientific Advisory Board member, in a press release on March 13, 2024.
In addition to the RSV program, Calder is applying its technology to Universal Influenza and Epstein-Barr virus vaccines.
The Nature Communications manuscript is linked here.