Search API
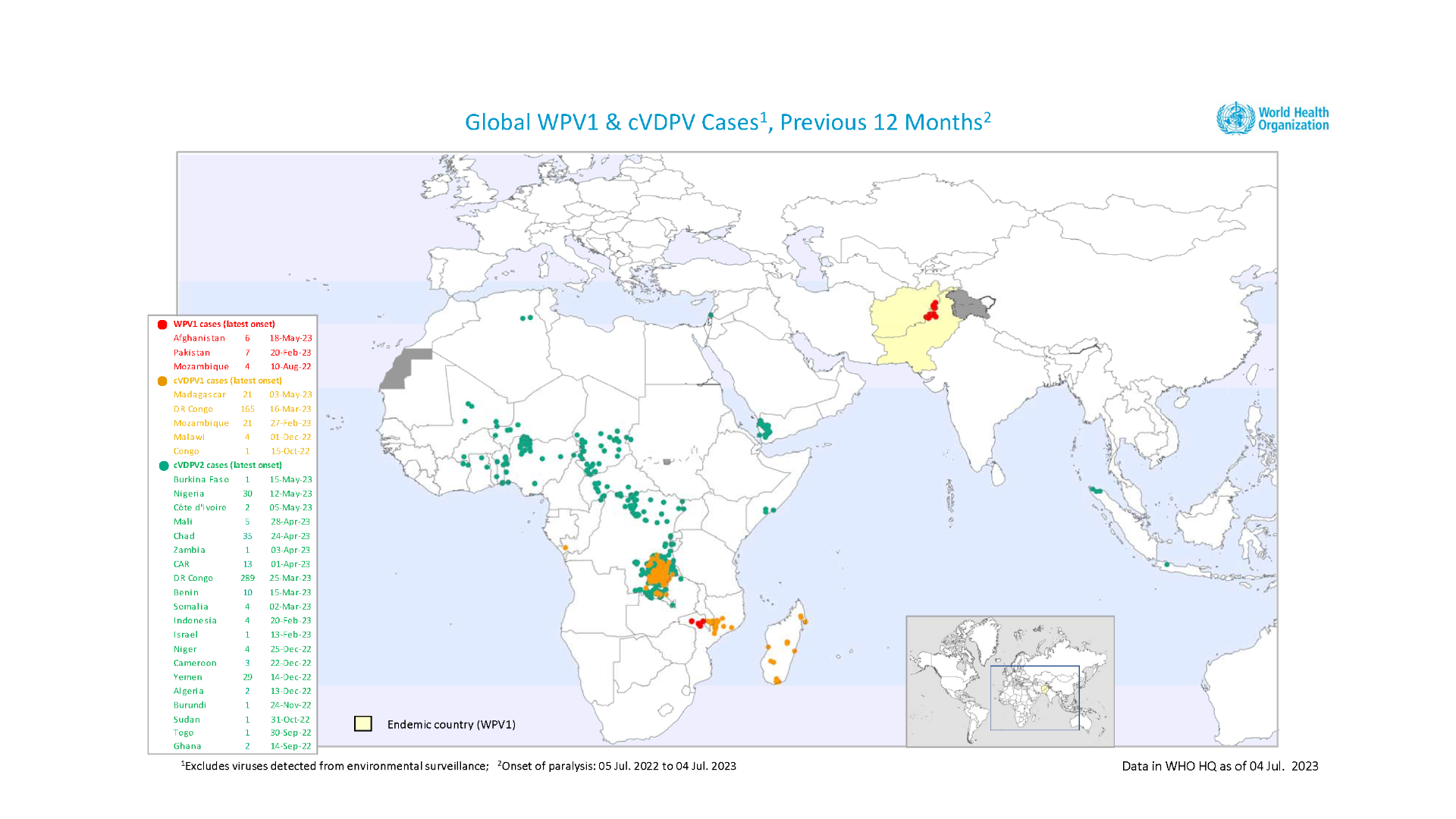
With 117 confirmed cases of circulating variant polioviruses in the WHO African Region this year, the Africa Regional Certification Commission recently urged countries to urgently address gaps in polio immunity to avert future outbreaks.
In recent years, polioviruses have paralyzed hundreds of African children, says the WHO.
On July 28, 2023, the WHO announced the detection of a circulating vaccine-derived poliovirus type 2 (cVDPV2) in two acute flaccid paralysis (AFP) cases and two asymptomatic healthy children community contacts from the Hagadera camp in Kenya.
The U.S. NIH says AFP surveillance is the standard for detecting cases of poliomyelitis in anyone under 15 years of age.
On July 25, 2023, Madagascar launched polio vaccinations for nearly 18 million children, adolescents, and adults in the priority regions of Analamanga, Vakinankaratra, and Alaotra Mangoro. Madagascar has reported 79 cases of cVDPV1 in 2023. Forty-five of them were cases of AFP, including 198 environmental samples.
On July 4, 2023, the Ministry of Health of the United Republic of Tanzania notified the WHO of the detection of cVDPV2. The virus was isolated from an AFP case in the Rukwa region, southwestern Tanzania.
To alert international travelers, the U.S. Centers for Disease Prevention and Control (CDC) included various African countries in its Global Polio Travel Health Advisory on July 10, 2023.
Furthermore, the U.S. was added to about thirty countries where polio was recently identified. In the U.S., poliovirus was confirmed in 2022 and 2023 in wastewater samples.
The CDC recommends polio vaccinations before visiting outbreak areas.
Merck & Co. today announced its human papillomavirus (HPV) vaccines sales increased 47% to reach $2.5 billion, and if you exclude the Impact of Foreign Exchange, sales actually grew by 53%.
Merck's GARDASIL 9® is a vaccine indicated in men and women, 9 through 45 years of age, to prevent cancers caused by the HPV. It has been available in the United States since late 2016.
The original GARDASIL® vaccine consists of 4 proteins of HPV types 6, 11, 16, and 18. is available in other countries.
According to the World Health Organization, HPV vaccination programs that began pre-pandemic reached the same number of women in 2022 as in 2019, with mean coverages reaching 67% in high-income countries and 55% in low- and middle-income countries.
Millions of men and women aged 27–45 may still benefit from HPV vaccination, says Merck.
"We continue to make great progress as we advance our broad and deep pipeline, raise the bar of innovation, and bring forward leading-edge science to save and improve lives around the world," said Robert M. Davis, chairman, and chief executive officer of Merck, in a press release issued on August 1, 2023.
HPV is a double-stranded DNA virus that belongs to the Papillomaviridae family. There are over 100 subtypes of HPV, characterized as high-risk or low-risk. And it is the most common sexually transmitted infection worldwide.
From a clinical perspective, HPV vaccinations reduce anal HPV infection and anal intraepithelial neoplasia (AIN).
A study published on May 31, 2023, concluded there is strong evidence for high vaccine efficacy against anal HPV infection and AIN in HIV-negative individuals vaccinated at age ≤26 years.

The U.S. Fish and Wildlife Service (FWS) Incident Command Team recently confirmed implementing conservation strategies to help California condors in light of the Highly Pathogenic Avian Influenza (HPAI) bird flu outbreak.
As of July 28, 2023, over twenty-one Condors have died related to HPAI infections this year.
In May 2023, the United States Department of Agriculture's Agricultural Research Service announced the emergency use of a HPAI vaccine candidate to prevent additional deaths of California Condors.
The California Condor Vaccination Trial continued will continue into September 2023.
Blood samples from 13 birds will be collected at 21 and 42-days following vaccination to evaluate the immune response from two different vaccination approaches.
The first sample will be collected on August 8.
From a recovery perspective, three condors were transferred to the release site in Arizona to reacclimate to their home. A release date will be determined based on their behavior and weather.
The fourth bird that survived also has immunity to HPAI and will be released later as he is currently re-growing molted flight feathers.
The California Condor Recovery Program continues to implement standard operations, and we are hopeful this will include the release of juveniles in 2023. However, due to the dynamic nature of HPAI outbreaks and logistics around potential future vaccinations, adjustments will be made accordingly, wrote the FWS.
The ongoing bird flu outbreak reached Europe, Asia, and Russia in 2023.
Furthermore, the U.S. government has already approved one bird flu vaccine (Audenz™) for people and invested in vaccine candidates should a pandemic occur.

60 Degrees Pharmaceuticals Inc. today announced that the Canadian Intellectual Property Office issued a patent on using novel tafenoquine regimens for malaria prevention in malaria-naive individuals, and it will remain valid until December 2, 2035.
The Company was issued a similar U.S. patent in 2019.
Tafenoquine is the active molecule in the Company's U.S. Food and Drug Administration-approved regimen for malaria prevention, ARAKODA®.
ARAKODA, an oral tablet containing 100 mg of tafenoquine base, is an anti-malarial indicated for malaria prevention in individuals 18 years and older.
As of July 31, 2023, travelers or individuals at risk of contracting malaria are prescribed 2 x 100 mg tablets once per day for three days (the loading phase) before travel, 2 x 100 mg tablets weekly for up to six months during travel, then 2 x 100 mg in the week following travel.
Travelers from, and residents of, Canada and the United States, are usually malaria naive because they have not previously contracted malaria and thus lack immunity to the disease.
During July 2023, malaria outbreaks have been reported in Florida and throughout Central America in countries such as Costa Rica. And numerous African countries have confirmed malaria outbreaks.
The bite of an infective female Anopheles mosquito spreads malaria. The disease can cause fever, chills, and flu-like illness. If it is not treated, it can cause severe complications and death, says the U.S. CDC.
Typically, about 2,000 malaria cases are diagnosed in the United States yearly.
In Africa, there are two malaria vaccines currently being administered.
And on April 26, 2023, the United States Patent and Trademark Office issued 60 Degrees a patent covering the use of tafenoquine as a treatment for COVID-19 disease.

GC Biopharma today announced that the U.S. Food and Drug Administration (FDA) accepted the Company's resubmission of the Biologics License Application for its GC5107B (Immune Globulin Intravenous) for patients with primary humoral immunodeficiency (PI).
GC5107B is a liquid solution containing 10% immunoglobulin G (100 mg/mL) for intravenous infusion, manufactured from pooled human plasma from U.S. donors.
The FDA's Prescription Drug User Fee Act target action date is January 13, 2024. If approved, GC Biopharma could provide more treatment options for patients with PI in the U.S. next year.
PI disease comprises a large, heterogeneous group of disorders resulting from inborn errors of immunity.
Patients with PI cannot mount an immune response to pathogens and can experience recurrent bacterial, viral, fungal, and protozoal infections as a result.
As of July 31, 2023, global estimates project that up to six million people may live with PI.
While the U.S. immunoglobulin market size is estimated at US$ 10.4 billion in 2022, there have been sporadic shortages, says GC Biopharma. The manufacturing process includes three steps to reduce the risk of virus transmission.
Note: Immune Globulin Intravenous products are not preventive vaccines.