Search API
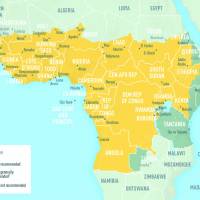
In 2023, the global Eliminate Yellow Fever Epidemics (EYE) secretariat led by the World Health Organization (WHO) coordinated preventive and responsive vaccination efforts.
As a result, about 62 million people in Africa received yellow fever vaccinations through EYE campaigns.
According to the WHO's Disease Outbreak News on March 20, 2024, a single yellow fever vaccination can provide lifelong protection and sustained immunity for around 90% of vaccinated individuals.
The WHO says yellow fever is an acute viral hemorrhagic disease. Cases can be difficult to distinguish from other viral hemorrhagic fevers such as arenavirus, hantavirus, or dengue. Related symptoms of yellow fever usually appear 3 to 6 days after the bite of an infected mosquito.
To notify international travelers, the U.S. Centers for Disease Control and Prevention advises against yellow fever vaccination when visiting countries with a low risk of virus exposure.
For Americans seeking protection from this mosquito-transmitted disease, the YF-VAX® vaccine is available at certified travel vaccine clinics and pharmacies in the U.S.
The CDC recently reported no locally acquired yellow fever cases in 2024.
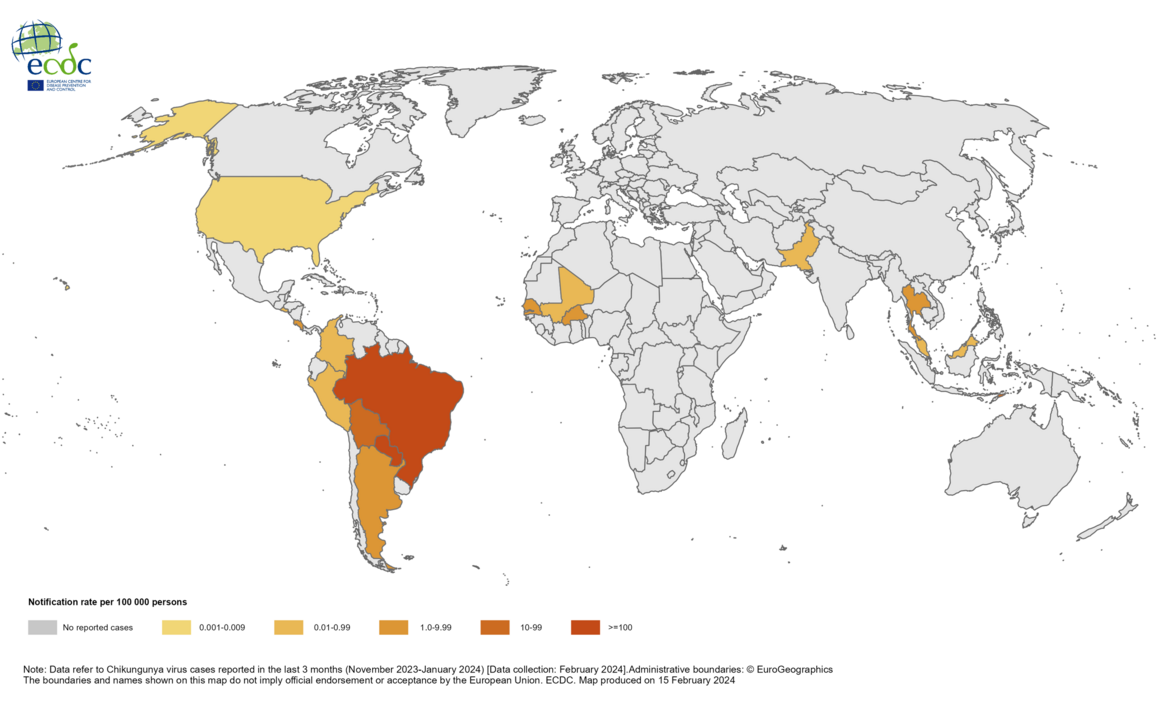
Millions of people's health is at risk by the mosquito-transmitted, vaccine-preventable chikungunya virus (CHIKV), but most Americans are unaware of its global impact.
In-person information about a newly approved chikungunya virus (CHIKV) vaccine will be shared at the Walter E. Washington Convention Center in Washington, D.C., from April 1-4, 2024.
Valneva SE, a France-based specialty vaccine company, today announced it will present its single-shot chikungunya vaccine for adults, IXCHIQ®, and participate in a panel discussion on efforts to eradicate chikungunya outbreaks at the 24th World Vaccine Congress.
XCHIQ®, the world's first and only chikungunya vaccine approved in the U.S., was recently recommended by the CDC's Advisory Committee on Immunization Practices for international travelers to at-risk areas.
As of March 21, 2024, CHIKV was identified in nearly 115 countries, primarily in the Region of the Americas. As of December 2023, approximately 460,000 CHIKV cases and 360 related deaths have been reported worldwide.
On the evening of April 2, Valneva will attend the Vaccine Industry Excellence Awards ceremony, where the vaccine is a finalist for the Best Prophylactic Vaccine award for IXCHIQ®.
And on April 3, Valneva's Chief Medical Officer, Dr. Juan Carlos Jaramillo, will participate in the "Vaccine Development and Efforts towards Eradicating Chikungunya" panel discussion.
For further details, contact Laetitia Bachelot-Fontaine, VP, Global Communications and European Investor Relations, at [email protected]

Valneva SE today reported its consolidated financial results for the year ending December 31, 2023, and provided several key corporate updates. Valneva's commercial portfolio is composed of three travel vaccines, IXIARO®/JESPECT®, DUKORAL®, and IXCHIQ®.
Valneva raised its 2024 product sales guidance to between €160 million and €180 million due to an improved outlook regarding the IXIARO® supply constraints anticipated in February 2024.
The Company made excellent progress across the R&D pipeline with the U.S. FDA's approval of the single-shot chikungunya vaccine IXCHIQ®, the world's first and only vaccine to address this significant unmet medical need.
In today's press release, Peter Bühler, Valneva's Chief Financial Officer, commented, "In 2023, Valneva successfully executed key strategic objectives despite a difficult economic environment."
"Our chikungunya vaccine IXCHIQ® became the world's first licensed chikungunya vaccine available to address this significant unmet medical need, and we also managed to surpass our pre-pandemic product sales."
Valneva will host a live webcast of its full-year 2023 results conference call on March 20, 2024, at 3 p.m. CET/10 a.m. EDT. The webcast will also be available on the Company's website. Please refer to this link: https://edge.media-server.com/mmc/p/hom3riyt.
Chikungunya is a viral disease transmitted by mosquitoes infected with the chikungunya virus. The World Health Organization says chikungunya outbreaks were identified in nearly 115 countries, primarily in the Region of the Americas, in 2023.
The U.S. CDC published today the Emerging Infectious Diseases, Early Release, Volume 30, Number 4—April 2024, that concluded where feasible, vaccination against hepatitis A, meningococcal disease (IMD), and mpox should be encouraged among at-risk groups and offered along with program services that target those groups.
On March 18, 2024, these CDC researchers wrote, 'We provide a descriptive, cross-sectional analysis of the concurrent outbreaks of hepatitis A and IMD in Florida in the context of an ongoing global mpox epidemic that also is disproportionally affecting MSM.'
'Through this analysis, we attempted to identify common and distinct features of each outbreak and synergistic factors that might have affected disease progression and control.'
'We observed a high percentage of concurrent HIV infection among hepatitis A, IMD, and most notably, mpox case-patients.'
The U.S. Food and Drug Administration has approved vaccines that can prevent certain sexually transmitted diseases (STDs) caused by infections from bacteria, viruses, or parasites. Various STD vaccines are available at health clinics and pharmacies in the U.S.

The Bill & Melinda Gates Medical Research Institute today announced that a Phase 3 clinical trial to assess the efficacy of the M72/AS01E tuberculosis (TB) vaccine candidate is now underway in South Africa.
At full capacity, the trial will include up to 20,000 participants, including people living with HIV, at up to 60 trial sites in seven countries.
If shown to be well-tolerated and effective, M72/AS01E could potentially become the first vaccine to help prevent pulmonary TB in adolescents and adults, the most common form of the disease.
Furthermore, M72/AS01E would be the first new TB vaccine in over a century.
While TB is one of the world’s deadliest infectious diseases, the only available vaccine is Bacille Calmette-Guerin (BCG), which dates back to 1921.
BCG vaccines initially targeted against TB, tuberculosis meningitis, and non-specific protective effects against respiratory tract infections and certain cancers.
Various reports indicate that the BCG vaccine offers inadequate protection for adolescents and adults against the pulmonary form of the disease, which is primarily responsible for transmitting the TB bacterium.

Merck today announced positive data from multiple Phase 3 studies evaluating V116, the company’s investigational, adult-specific 21-valent pneumococcal conjugate vaccine.
Across the clinical studies presented at the 13th Meeting of the International Society of Pneumonia and Pneumococcal Diseases in Cape Town, South Africa, on March 19, 2024, V116 was shown to be immunogenic for all 21 serotypes covered by the vaccine in a variety of adult populations, including those who had not previously received a pneumococcal vaccine (pneumococcal vaccine-naïve), those who had previously received a pneumococcal vaccine (pneumococcal vaccine-experienced) and those with an increased risk of pneumococcal disease, including people living with human immunodeficiency virus.
In all STRIDE studies presented at the meeting, V116 also elicited higher immune responses than the studied comparators for the serotypes unique to V116.
Several studies presented today were included in the filing submission to the U.S. Food and Drug Administration (FDA). The FDA granted V116 priority review with a Prescription Drug User Fee Act of June 17, 2024.
If approved by the FDA, V116 would be the first pneumococcal conjugate vaccine specifically designed for adults.