Search API
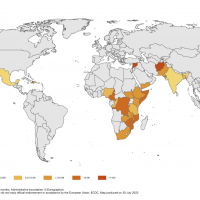
Bavarian Nordic A/S today announced positive topline results from a Phase 3 clinical trial of its virus-like particle (VLP)-based chikungunya virus vaccine candidate, CHIKV VLP (PXVX0317).
The results up to day 22 post-vaccination showed that CHIKV VLP was highly immunogenic in healthy adolescents and adults, as demonstrated by the strong induction of chikungunya-neutralizing antibodies in 98% of vaccinees in the active group.
The strong neutralizing antibody titres were equal to or exceeded the threshold agreed upon with authorities as a seroprotection marker, meeting the study's primary objectives.
Importantly, CHIKV VLP induced significant neutralizing antibodies in 97% of the subjects at two weeks post vaccination, confirming a rapid onset of protective immunity levels.
These responses were robust and durable, as 86% of the subjects had seroprotective levels of neutralizing antibodies six months post vaccination.
"We are highly encouraged by the positive topline results now demonstrated in both Phase 3 studies of our chikungunya vaccine candidate. Our focus remains to finalize the studies and prepare for regulatory submissions next year," said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release on August 6, 2023.
"With a fast and durable response, our vaccine has the potential to be the best in class to prevent chikungunya infections in adolescents to elderly adults."
"Chikungunya can often result in a severe and incapacitating disease affects large parts of the world, and with international travel on the rise again, our CHIKV vaccine offers a significant opportunity to address this large unmet medical need."
These results help form the basis for submission of a Biologics License Application to the U.S. Food and Drug Administration and a Marketing Authorization Application to the European Medicines Agency in 2024 to support potential launch of the vaccine in 2025.
The U.S. CDC published a Morbidity and Mortality Weekly Report (MMWR) that concluded post-authorization safety data after receipt of a primary Novavax COVID-19 vaccine dose is limited by the low number of doses administered (0.01% of total COVID-19 vaccines administered), available data are consistent with those from preauthorization clinical trials.
And no new safety concerns were identified, wrote the CDC on August 4, 2023.
This MMWR stated from July 13, 2022–March 13, 2023, a total of 69,227 Novavax doses were administered to persons in the U.S., and 230 reports of adverse events after vaccination were received by the Vaccine Adverse Event Reporting System (VAERS).
Among the 230 reports received, 19 (8.3%) were classified as serious, and no deaths were reported after vaccination.
Serious reports included one case of thrombosis, two of pericarditis, one of Guillain-Barré syndrome, and two of seizure.
The remaining serious reports described chest pain, arrhythmia, sickness, hospitalization, adverse event not otherwise specified, balance disorder, peripheral neuropathy aggravated, and vaccine failure.
Limitations of this analysis include reporting biases and inconsistency in the quality and completeness of reports to VAERS.
Furthermore, VAERS data generally cannot be used to determine whether a vaccine caused an adverse event.
In addition, approximately one-half of the reports representing adverse events of special interest lacked medical records for CDC review.
Novavax COVID-19 Vaccine Adjuvanted (Nuvaxovid™, CovoVax™, NVX-CoV2373) was the first protein-based vaccine engineered from the genetic sequence of the SARS-CoV-2 beta coronavirus.

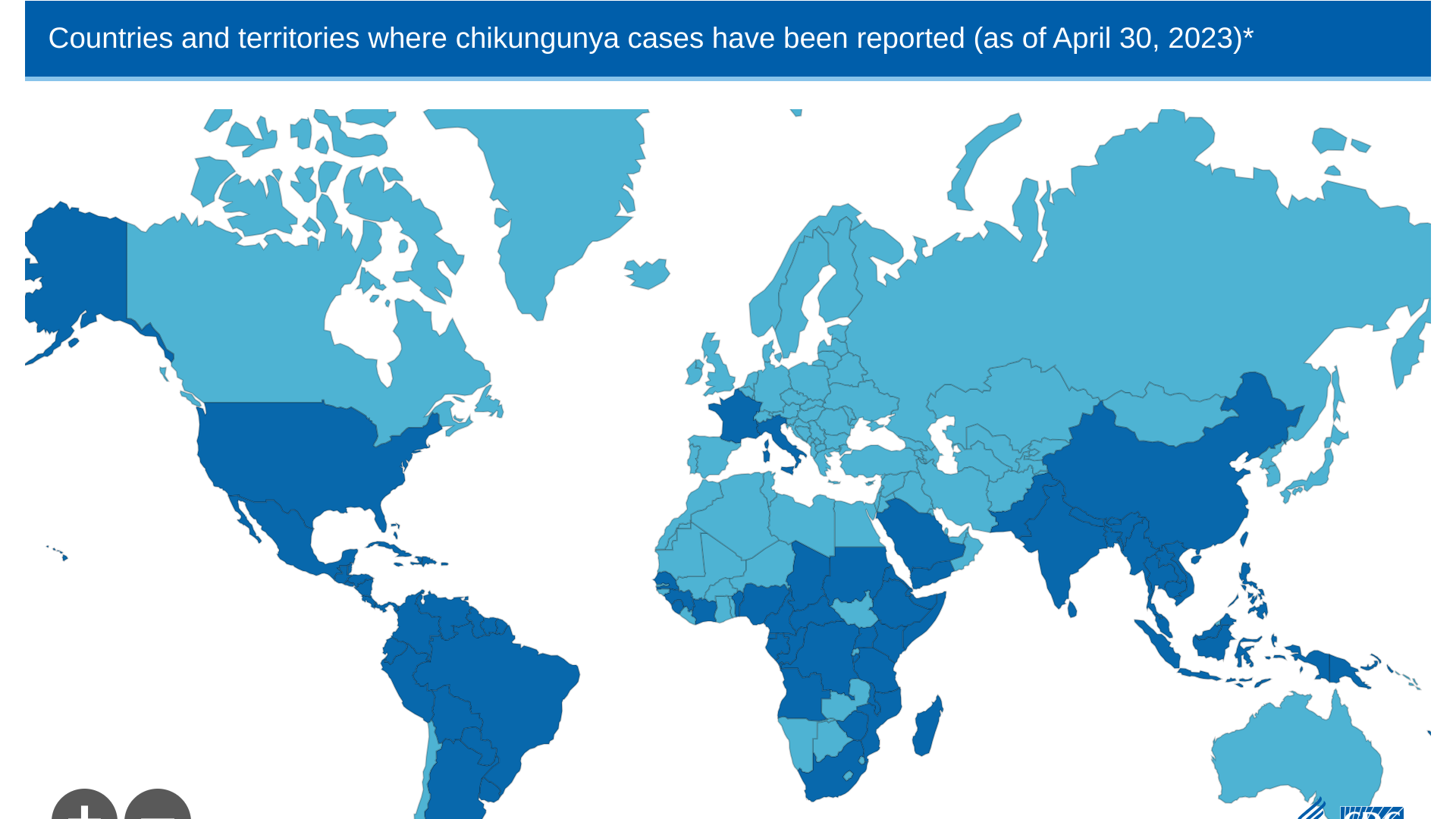
According to the U.S. Centers for Disease Control and Prevention (CDC), measles is an ongoing global health risk.
As of early July 2023, health officials in 38 countries reported large measles outbreaks.
The top ten measles outbreaks were led by India, with 67,592 cases, as of July 18, 2023.
The CDC reissued a Level 1, Practice Usual Precautions Travel Health Advisory to alert international travelers of this risk. The CDC says measles is a risk if a person is not fully vaccinated two weeks before departure or has not had measles in the past.
The CDC says international travelers, including infants 6–11 months of age and preschool-aged children, should be fully vaccinated against measles with a measles-mumps-rubella (MMR) vaccine.
This CDC web tool empowers people to determine whether or not they need additional measles vaccination before departure.
MMR vaccines are generally available in health clinics and community pharmacies in the U.S.
Measles is highly contagious, even on airplanes. Travelers should seek medical care if they develop a rash, high fever, cough, runny nose, or red, watery eyes. Vitamin A deficiency is a recognized risk factor for severe measles infections.
Travelers with suspected measles should notify the healthcare facility before visiting so staff can implement precautions to prevent the spread within the facility.
In the U.S., the CDC has reported 19 measles cases in thirteen U.S. jurisdictions as of August 3, 2023. In 2022, there were 121 measles cases.
The U.S. Transportation Security Administration (TSA) recently reported the number of air travelers passing through airport security has returned to pre-pandemic levels in July and early August 2023.
About 2.4 million people are being screened by TSA security each day.
And according to new data from the TSA, screening can be measured in minutes if you are a TSA PreCheck® member.
During July 2023, about 89% of air passengers waited less than 5 minutes to be processed by TSA PreCheck.
TSA PreCheck is a Trusted Traveler Program offering expedited security screening services at 200 airports in the U.S. To learn more about TSA PreCheck, visit the TSA PreCheck page or the TSA PreCheck® FAQ webpage.

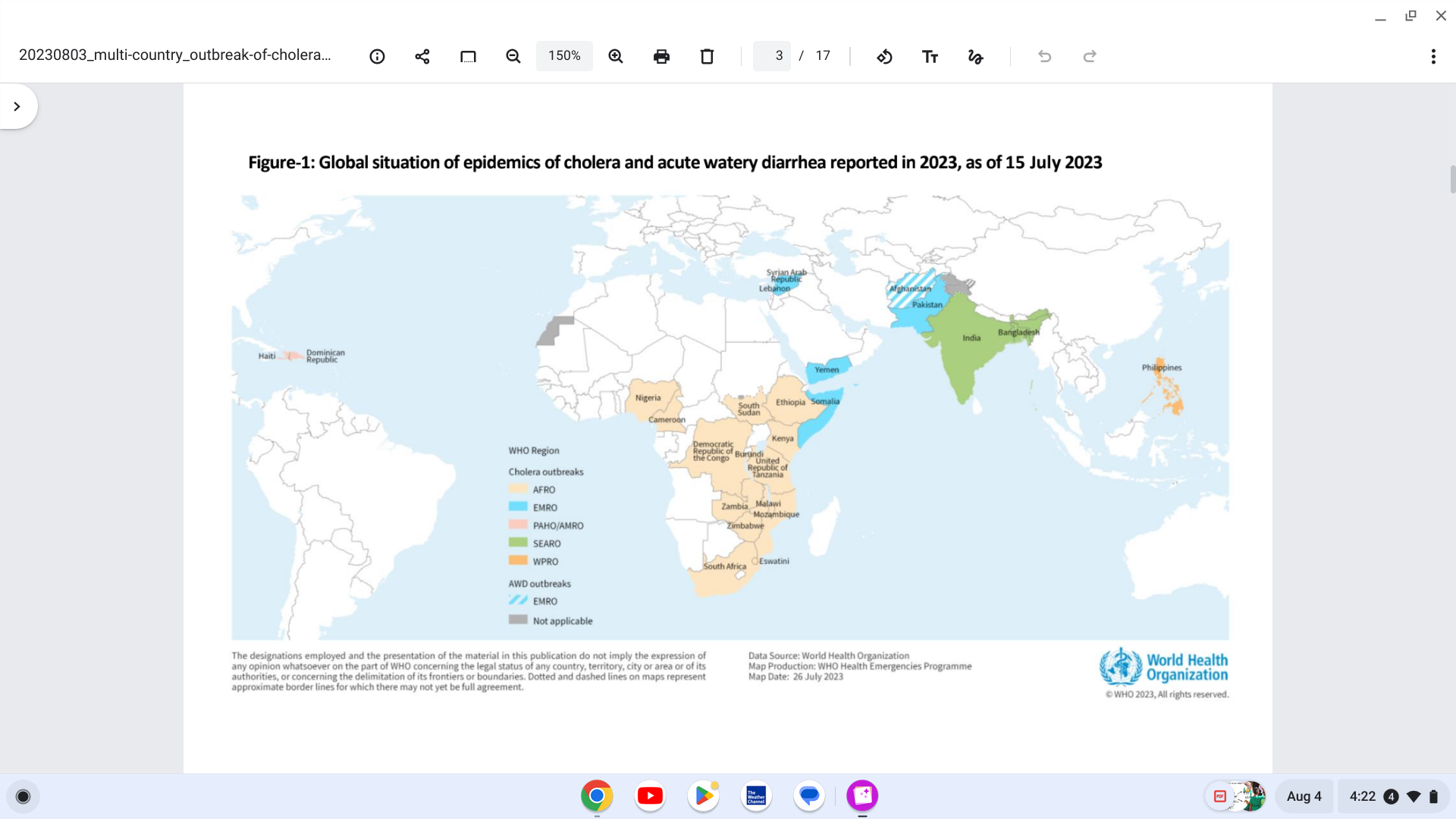
The World Health Organization's (WHO) latest multi-country cholera outbreak situation (#5) says that Based on the large number of outbreaks and their geographic expansion, WHO continues to assess the risk at the global level as very high.
As of August 4, 2023, twenty-five countries have reported cholera outbreaks since the beginning of 2023.
India became the 25th country on May 15, 2023.
The WHO African Region remains the most affected region, with 14 countries reporting cholera cases this year.
In the Region of the Americas, Haiti reported 54.826 cholera cases last year.
The overall capacity to respond to multiple and simultaneous outbreaks continues to be strained due to the global lack of resources, including shortages of the Oral Cholera Vaccine (OCV).
Since the start of 2023 and as of July 24, 2023, a total of 49.9 million OCV doses have been requested, of which 19.3 million (39%) have been approved for 11 countries. The available (not yet allocated) global OCV stockpile was 2.7 million doses.
In the current outbreak context, only one-dose courses have been validated and implemented in these reactive campaigns. Doses for preventive campaigns cannot be supplied due to the low global stockpile, says the WHO.
In the U.S., the CDC recommends that adults traveling to areas with active cholera transmission get vaccinated with a newly licensed cholera vaccine, Bavarian Nordic's Vaxchora®.
This OCV is indicated for active immunization against disease caused by Vibrio cholerae serogroup O1 in adults.
According to Vaxchora's manufacturers, the vaccine has limited availability in 2023.
Another vaccine. Valneva SE's Dukoral® is administered with a buffer solution that, for adults, requires 150 ml of clean water. Dukoral can be given to all individuals over the age of 2 years.
The Access to Advanced Health Institute (AAHI) today announced that it has received an $18 million award from the National Institutes of Health (NIH) to develop a temperature stable, single-dose, RNA chikungunya vaccine candidate.
The NIH award disclosed on August 3, 2023, supports the development, preclinical testing, and human clinical evaluation of a vaccine that meets an increasingly urgent need for a reliable, abundant supply.
Chikungunya is a viral disease transmitted to humans through the bites of mosquitoes infected with the chikungunya virus (CHIKV), says the U.S. CDC.
Chikungunya outbreaks are significant causes of morbidity and mortality in Asia, Africa, and Latin America, for which no vaccine is currently approved.
From 2006–2021, 4,590 chikungunya cases in travelers were reported in the U.S.
Several vaccine candidates are conducting late-stage clinical trials, such as Valneva SE's VLA1553, a monovalent, single-dose, live-attenuated vaccine candidate.
AAHI's approach to an RNA vaccine against chikungunya differs from the RNA vaccines the U.S. FDA currently approves to prevent other diseases.
“This project will demonstrate the use of RNA vaccine technology to avoid some of the classic manufacturing challenges in the large-scale manufacture of live-attenuated vaccines,” said Emily Voigt, Ph.D., Principal Scientist, AAHI RNA Platform Lead, and Co-Principal Investigator for the award, in a press release.
Unlike other RNA vaccines, this candidate will generate a live "attenuated" virus that could induce strong and long-lasting immune protection against this mosquito-borne disease.
The new 5-year project builds upon work supported by the NIH (R43AI127053) for AAHI's proof-of-concept ground-laying work, which demonstrated that a liquid presentation of this live-attenuated chikungunya RNA vaccine candidate elicited strong immune responses in animals after a single dose, protecting them from mortality and joint swelling after being challenged with the virus (Voigt et al. 2021).
AAHI is a nonprofit biotech research institute located in Seattle, Washington, that combines the high-quality science of an academic research organization with the product development capabilities of a biotech company to help combat some of the world's deadliest diseases, including infectious diseases