Search API
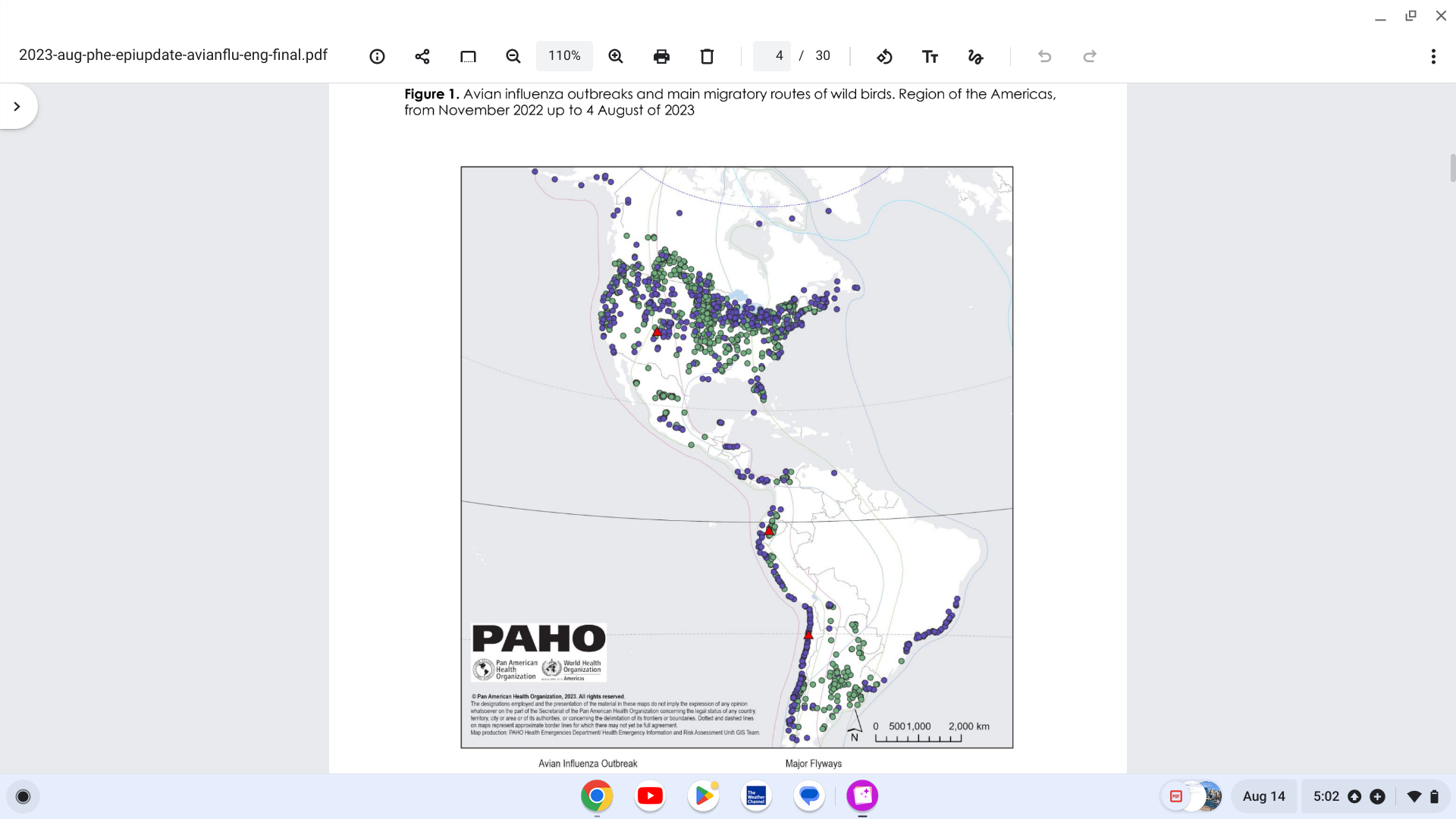
The Pan American Health Organization (PAHO) recently stated that the detection of avian influenza (bird flu) outbreaks in 15 countries in Latin America and the Caribbean has never been recorded before.
These bird flu outbreaks are mainly located in areas along the Pacific Flyway.
As of epidemiological week #31 of 2023, the PAHO reported authorities in Argentina, Bolivia, Brazil, Canada, Chile, Colombia, Costa Rica, Cuba, Ecuador, Guatemala, Honduras, Mexico, Panama, Peru, Uruguay, the USA, and Venezuela have detected outbreaks of Highly pathogenic avian influenza A(H5N1) viruses in domestic birds, farm poultry and/or wild birds, and in mammals.
Among the mammals identified as of August 9, 2023, red foxes and skunks were the most frequently affected in North America and fur seals in South America. For example, Argentina's Senasa reported on August 11, 2023, the country's first avian flu detections in sea lions.
And in Chile, about 16,000 sea lions have died due to HPAI infections this year.
Whenever avian influenza viruses circulate among poultry, there is a risk of sporadic occurrence of human cases due to exposure to infected poultry or contaminated environments.
From 2003 to July 14, 2023, twenty-three countries reported 878 human cases of influenza A(H5N1) infection, including 458 deaths (case fatality ratio 52%), says the PAHO.
While there are ongoing investments in bird flu vaccines for mammals and people, the U.S. CDC reaffirms the annual flu shot is not designed to protect people from HAPI viruses.
The United States has maintained the elimination of measles since 2000. However, measles outbreaks have recently occurred when people travel to and from the U.S., especially when travelers are unvaccinated or under-vaccinated against measles.
The CDC updated its list of the top ten measles outbreaks on August 10, 2023, indicating India has reported over 57,000 measles cases during the past year.
And they reissued a Level 1 Travel Health Notice in late June 2023, confirming a global measles outbreak.
The U.S. Centers for Disease Control and Prevention (CDC) stated it would conduct a Clinician Outreach and Communication Activity (COCA) webinar on August 17, 2023, focused on eliminating measles in the U.S.
This effort requires continued investment in the measles vaccination program,s which are instrumental to achieving elimination.
Additionally, healthcare providers and public health authorities need to remain vigilant to rapidly recognize measles and take steps to mitigate the spread within communities for continued measles elimination. Healthcare providers should consider measles a diagnosis in anyone with a fever (≥101°F or 38.3°C) and a generalized maculopapular rash with cough, coryza, or conjunctivitis who has recently been abroad, especially in countries with ongoing outbreaks.
Furthermore, the CDC urges all healthcare providers to ensure their patients are current on measles, mumps, and rubella vaccination.
During this COCA Call, presenters will discuss the history of measles in the U.S., review clinical presentation and diagnosis of measles infection, review how to report suspected cases to public health agencies and outline recommendations for measles vaccination in the U.S.
When: Thursday, August 17, 2023, 2:00 PM – 3:00 PM ET; Webinar Link: https://www.zoomgov.com/j/1603132944; Webinar ID: 160 313 2944; Passcode: 532989.
In the U.S., various measles vaccines are generally available at health clinics and community pharmacies.

Valneva SE today announced that the U.S. Food and Drug Administration (FDA) has revised the Prescription Drug User Fee Act (PDUFA) action date for the Biologics License Application (BLA) for VLA1553, a monovalent chikungunya virus vaccine candidate.
Valneva is committed to working with the FDA in its ongoing BLA review and potentially delivering the world's first chikungunya vaccine.
The previously communicated end of August PDUFA has been adjusted to the end of November 2023.
Valneva stated on August 14, 2023, the FDA extended the PDUFA date to allow sufficient time to align and agree on the phase 4 program necessary under the accelerated approval pathway.
Furthermore, no additional clinical data have been requested for the FDA approval process.
Juan Carlos Jaramillo, Chief Medical Officer of Valneva, said in a press release, "We appreciate and take pride in the fact that our BLA for VLA1553 if approved, will represent the first vaccine candidate to be approved under the accelerated approval pathway in an outbreak disease, and hence the necessary Phase 4 activities will set a future standard."
The Company reconfirms its previous guidance for potential BLA approval, initial launch, and potential award of a priority review voucher in 2023. This PDUFA extension does not impact Valneva's current regulatory submission in Canada or its planned submission with the European Medicines Agency.
VLA1553 is a single-dose, live-attenuated chikungunya vaccine candidate based on an infectious clone (CHIKV LR2006-OPY1) attenuated by deleting a gene encoding the non-structural replicase complex protein nsP3 protection against various Chikungunya virus phylogroups and strains.
Valneva's VLA1553 vaccine candidate is designed for prophylactic, active immunization against Chikungunya in humans over 1-year-old.
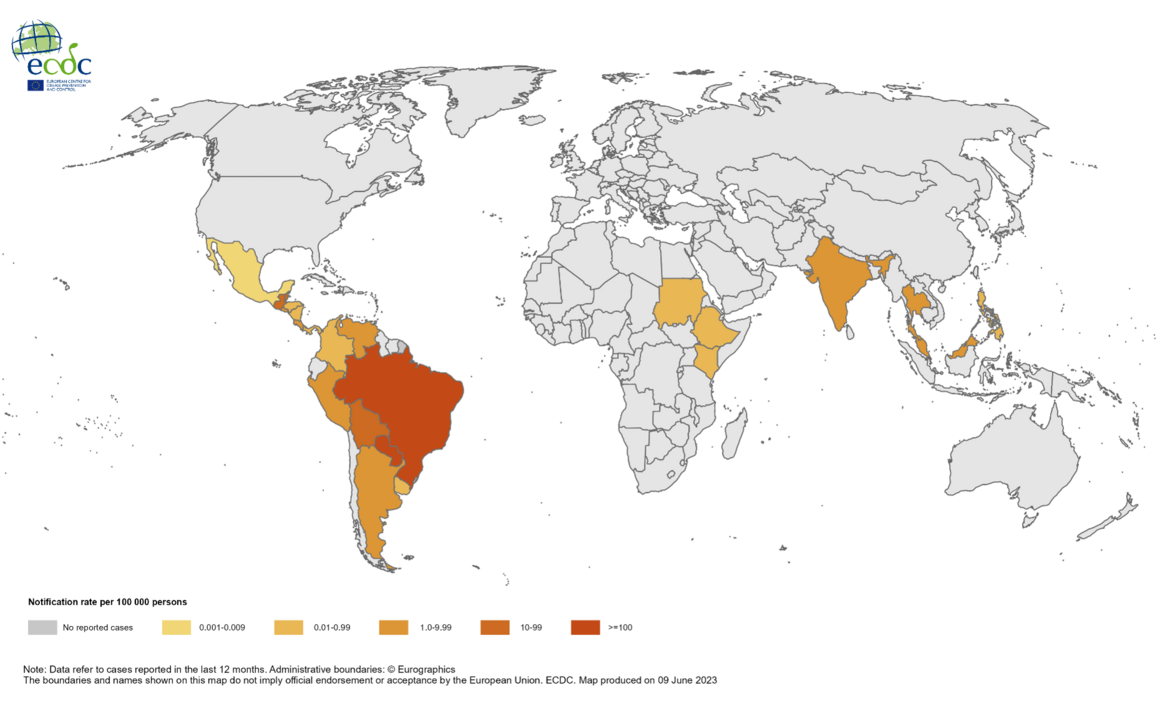
Chikungunya is a viral disease transmitted to humans through the bites of mosquitoes, causing outbreaks in 2023.
The European Centre for Disease Prevention and Control reported that as of July 26, 2023, approximately 300,000 cases and over 300 deaths have been reported worldwide due to Chikungunya virus disease.
Since rabies is a serious ongoing public health concern, the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) announced on August 4, 2023, its annual distribution of RABORAL V-RG®, an oral rabies vaccine (ORV) bait, would be distributed in select areas in the eastern United States to prevent the spread of raccoon rabies.
ORV baits are coated with a fishmeal attractant and are packaged in two-inch plastic sachets or one-inch square cubes.
The RABORAL V-RG vaccine is safe for many animals, including domestic dogs and cats. Humans and pets cannot get rabies from contact with the baits.
If adults or children come in contact with baits, immediately rinse the contact area with warm water and soap, says the APHIS.
While raccoons and dogs are high-risk rabies carriers, wild, infected bats are the leading cause of rabies in the U.S.
Rabies is found in more than 150 countries and territories, say the World Health Organization. And rabies infections are almost always fatal once symptoms appear, but deaths can generally be prevented with appropriate therapies.
The U.S. Centers for Disease Control and Prevention updated its recommendations for rabies preexposure prophylaxis for humans in 2022, now endorsing a two-dose program.
In the U.S., rabies vaccines are available in 2023.

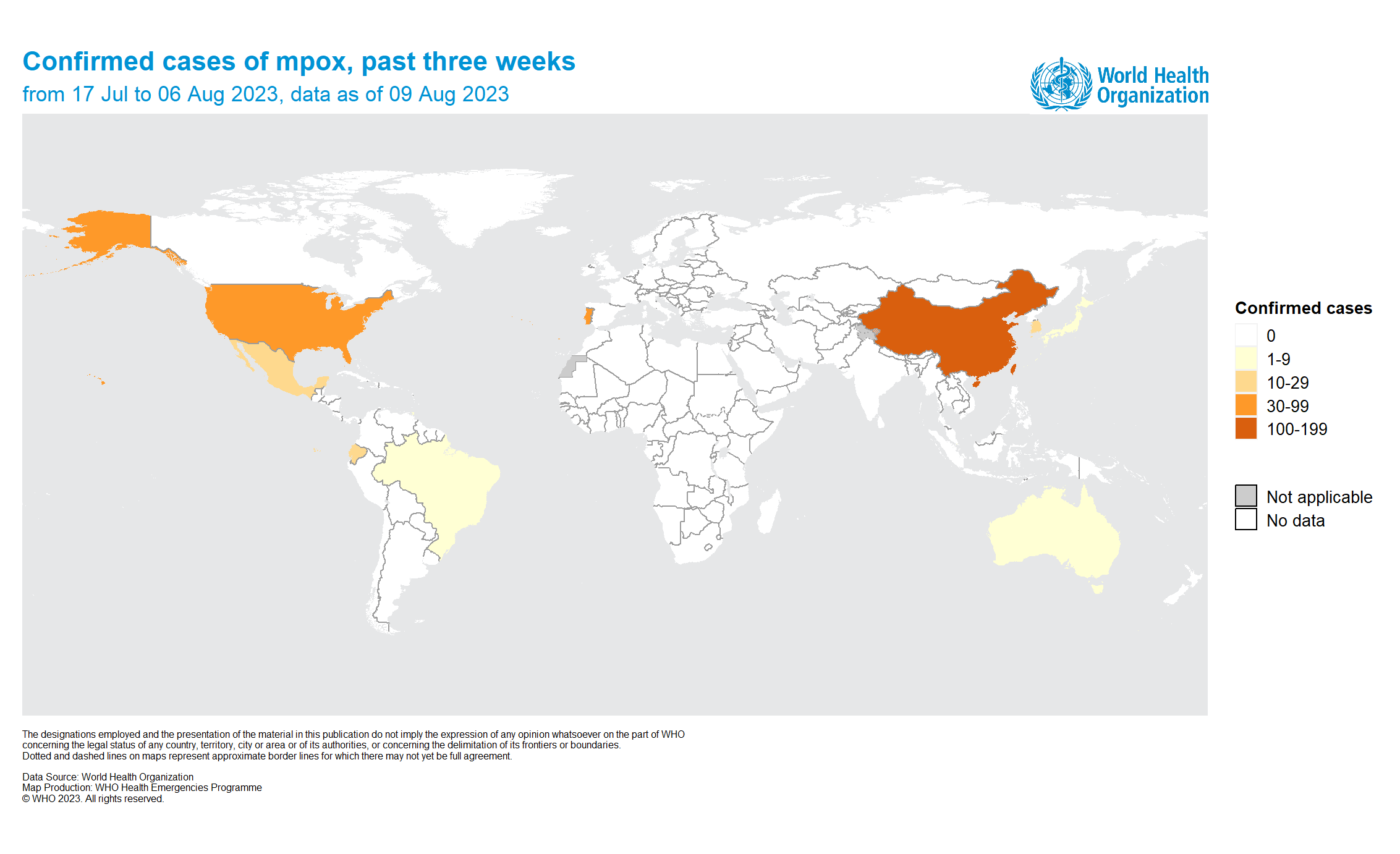
The World Health Organization (WHO) today reported that 15 countries had reported mpox outbreaks in the past three weeks.
As of August 9, 2023, the highest increase in mpox cases was reported in Mexico.
The most affected region was the Western Pacific Region, where 915 cases, the Region of the Americas (395 cases, 9 deaths), and the African Region (227 cases).
The ten most affected countries since May 2022 are the United States of America (30,446), Brazil (10,967), Spain (7,560), France (4,150), Colombia (4,090), Mexico (4,045), Peru (3,812), the United Kingdom (3,771), Germany (3,694), and Canada (1,496).
These countries account for 82.9% of the cases reported globally, says the WHO.
As of August 11, 2023, mpox vaccines remain available in most impacted countries.
Vaxxinity, Inc. today announced The Lancet's eBioMedicine published results of Phase 2a clinical trial stating that UB-311 "was safe and well-tolerated," with early clinical data demonstrating a trend for slowing cognitive decline in mild Alzheimer's disease (AD).
In this 78-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter Phase 2a, Vaxxinity-funded study, UB-311 was reported to elicit a robust, rapid, and titrated antibody response to Aβ.
And UB-311 was generally well-tolerated, with no cases of ARIA-E and limited cases of asymptomatic ARIA-H.
"This publication supports the innovative work that we and our collaborators are conducting to advance UB-311 for the potential treatment, and even prevention, of Alzheimer's disease," said Mei Mei Hu, CEO of Vaxxinity, in a press release on August 10, 2023.
"Imagine expanding the addressable patient population of beta-amyloid immunotherapies by multiple orders of magnitude, potentially over 1,000x, and delivering life-changing medicine at a fraction of the cost. That is our vision for UB-311 and the potential power of active immunotherapies."
AD is the most common form of dementia, is a progressive neurodegenerative disorder that slowly destroys memory and cognitive skills and eventually the ability to carry out simple tasks.
UB-311 is a synthetic, peptide-based active immunotherapy that targets toxic beta-amyloid (Aβ) oligomers and fibrils and oligomers.
Two passive immunotherapies – monoclonal antibodies targeting Aβ – have recently been authorized by the U.S. FDA, validating Aβ as a target for disease-modifying immunotherapies of AD.
However, these passive immunotherapies have been associated with amyloid-related imaging abnormalities (ARIA), which can present as vasogenic edema or sulcal effusion (ARIA-E) or as hemosiderin deposits such as micro hemorrhages and superficial siderosis (ARIA-H).
Although the trial was not powered to make conclusions about efficacy, secondary efficacy outcomes on cognitive, functional, behavioral, and global assessments such as ADAS-Cog, MMSE, ADCS-ADL, and CDR-SB were evaluated.
Trends of slowing disease progression were observed across key cognitive and functional measures for UB-311-treated versus placebo-treated participants over 78 weeks of observation, including a 48% slowing of decline on CDR-SB in the UB-311 quarterly boosting group.
Furthermore, the U.S. FDA-licensed mAbs require IV infusions every two weeks and are priced at $26,500 annually, not including the cost of administering them or monitoring for ARIA.
In contrast, UB-311 has the potential to offer multiple competitive advantages, including lower rates of ARIA-E, improved convenience through less frequent dosing and ease of administration through intramuscular injection, and overall improved accessibility and cost-effectiveness for patients and health systems.
As of August 10, 2023, the U.S. FDA had not approved an Alzheimer's vaccine candidate.