Search API
Although human infections with Avian Influenza HPAI A(H5N1) virus (Bird Flu) are rare, having unprotected exposure to any infected animal poses a risk of infection.
The U.S. Centers for Disease Control and Prevention (CDC) issued guidelines on March 29, 2024, to prevent exposure to this virus.
These guidelines include using personal protective equipment, testing, antiviral treatment, patient investigations, monitoring exposed individuals, and administering antiviral chemoprophylaxis to those who have been exposed.
Currently, the CDC considers the human health risk to people in the U.S. from Bird Flu viruses to be low.
These CDC recommendations are based on information available as of March 2024 and will be updated as necessary when new information becomes available.
Influenza A viruses infect birds' respiratory and gastrointestinal tracts, causing them to shed the virus in their saliva, mucous, and feces. Human infections with avian influenza A viruses can happen when enough virus gets into a person's eyes, nose, or mouth or is inhaled.
Illnesses in people from HPAI A(H5N1) virus infections have ranged from mild to severe disease, resulting in death, says the CDC.
As of March 31, 2024, the U.S. FDA approved bird flu vaccines for people, such as CSL Seqirus Inc. Audenz™ (aH5N1c), but they are not commercially available.
Additionally, the U.S. government has previously invested in developing various avian influenza vaccines and candidates.

Brazil's health officials are concerned about an unprecedented number of dengue cases reported in early 2024—more than one million. Traditionally, Brazil's dengue cases peak between March and May.
As reported by the AP, Brazil declared public health emergencies in Acre, Minas Gerais, Goias, and the Federal District in February 2024 to increase awareness.
According to the Municipal Health Secretariat of Rio de Janeiro, the "Against Dengue Every Day" campaign included the distribution of repellents, stickers, bandanas, and hats with warnings about the mosquito-transmitted disease.
Rio is a very popular vacation destination for events such as Carnival, receiving about 2 million foreign tourists.
The AP also reported that a section of Rio deployed the Wolbachia technique, also known as the Incompatible Insect Technique, and is seeing initial, positive results.
This biological method uses Wolbachia-infected mosquitoes to reduce the number of mosquitoes that spread vector-borne diseases. This method has been tested in the United States since 2016, initially in Miami, Florida.
In addition to genetically modifying local mosquitoes, Brazil has been offering dengue vaccinations.
Brazil became the first Latin American country to include the second-generation QDENGA® (TAK-003) vaccine in its public health system. The government plans to administer over 5 million doses in 2024.
This dengue vaccine does not require pre-admission testing.
To expand access, Biological E. Limited committed in February 2024 to manufacturing up to 50 million QDENGA doses annually, accelerating the vaccine's owner Takeda's ability to deliver 100 million doses annually by 2030.
As of March 30, 2024, QDENGA is unavailable in the United States.

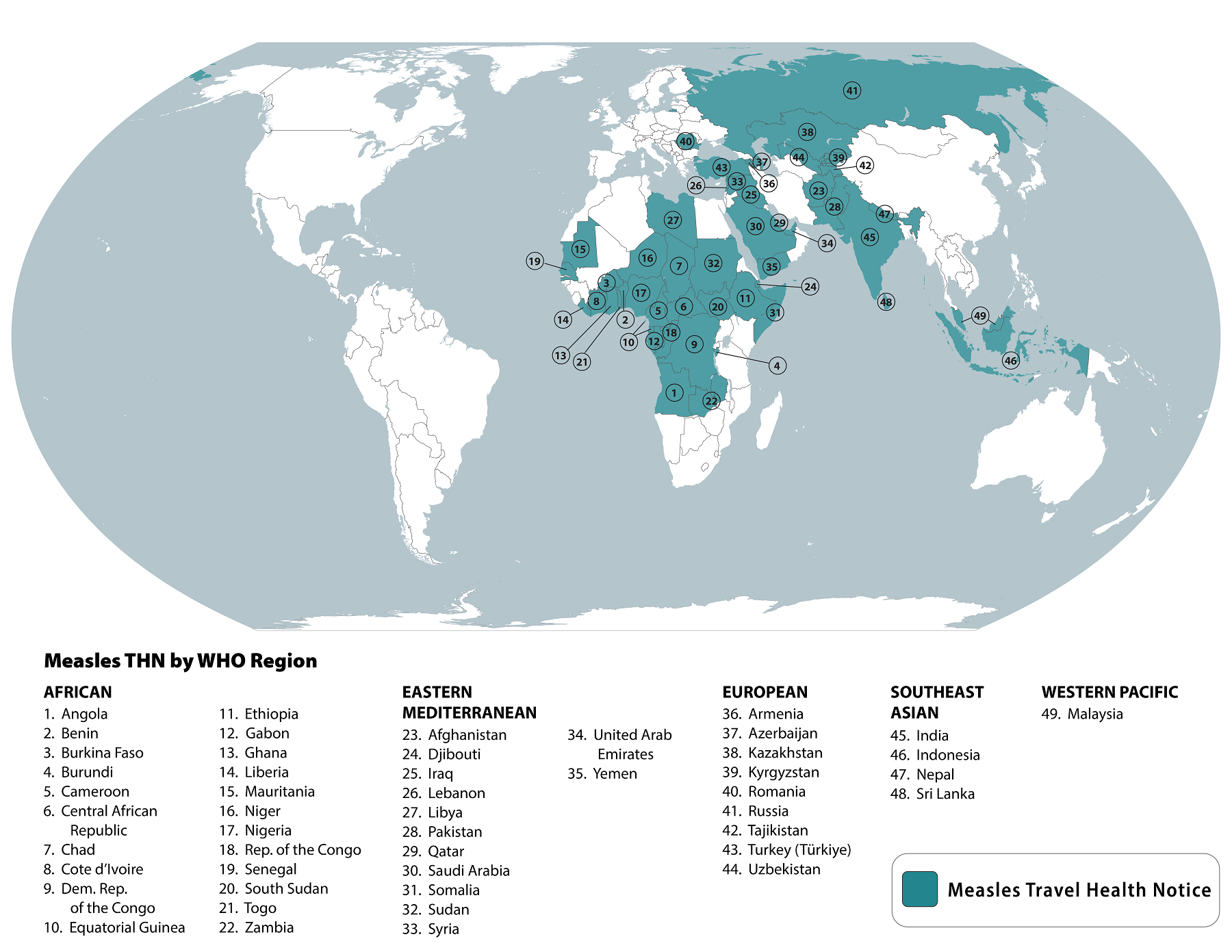
Measles outbreaks remain an ongoing health risk worldwide, and various health officials are taking action to limit this vaccine-preventable disease.
According to the U.S. Centers for Disease Control and Prevention (CDC), 49 countries are now reporting measles outbreaks.
The CDC stated on March 22, 2024, that travelers to these countries, including the United States, are at risk of measles if they have not been fully vaccinated at least two weeks before departure or have not had measles in the past and travel internationally.
The CDC says the majority of measles cases imported into the United States occur in unvaccinated U.S. residents who become infected during international travel.
For example, the Chicago (Illinois) Department of Public Health has reported 52 measles cases in unvaccinated people at a local shelter this year.
According to the CDC's vaccination recommendations for international travel, all travelers, including most children, should be fully vaccinated against measles with a measles-mumps-rubella vaccine.
Measles vaccines are generally offered at health clinics and community pharmacies in the U.S.
Dr. Reddy's Laboratories Ltd. today announced that it has entered into an exclusive partnership with Sanofi Healthcare India Private Limited to promote and distribute its vaccine brands across private markets in India.
Under the arrangement confirmed on March 27, 2024, Dr. Reddy's will have exclusive rights to promote and distribute Sanofi's well-established and trusted pediatric and adult vaccine brands Hexaxim®, Pentaxim®, Tetraxim®, Menactra®, FluQuadri®, Adacel®, and Avaxim® 80U.
Sanofi will continue to own, manufacture, and import these brands into India.
These brands saw combined sales of approximately $51 million as of February 2024.
M.V. Ramana, Chief Executive Officer, Branded Markets (India and Emerging Markets), Dr. Reddy's, commented in a press release, "We are happy to have the opportunity to leverage our strengths in promotion and distribution to considerably expand engagement with healthcare professionals and help widen access of Sanofi's well-established and trusted vaccine brands in India."
"The portfolio now gives Dr. Reddy's a strong presence in the vaccine segment, propelling us to the second position among vaccine players in India. Through each product and partnership, we aim to serve over 1.5 billion patients by 2030."
Dr. Reddy's was established in 1984 and has become a global pharmaceutical company headquartered in Hyderabad, India.
The U.S. CDC recommends visiting your healthcare provider at least a month before visiting India to get any vaccines or medicines you may need.
Additionally, the CDC included India in its recent Global Measles travel advisory.
Vaccines to protect people against Zaire Ebolavirus outbreaks have been used during outbreaks over the past few years.
According to the World Health Organization, two Ebola vaccines are available in 2024.
A recent study has confirmed that the prime-boost Ebola vaccine regimen is safe and effective for children and adults.
This phase 2 study assessed the long-term immunogenicity of the MVA-BN-Filo vaccine regimen and the safety of an immune memory response to an Ad26.ZEBOV booster vaccination.
These researchers concluded, in a paper published on March 26, 2024, that the vaccine regimen and booster dose were well tolerated.
These researchers wrote that a similar and robust humoral immune response was observed for participants boosted one year and two years after the first dose, supporting the use of the regimen and flexibility of booster dose administration for prophylactic vaccination in at-risk populations.
The other recommended Ebola vaccine is Merck's Ervebo®, which was approved in 2019.
However, no vaccines have been approved to protect people against the Sudan Ebolavirus.
In 2024, ten years after the West African Zaire Ebola outbreak, the World Health Organization updated its guidelines on infection prevention and control for Ebola disease.

In 2016, the Philippine Department of Health implemented a dengue vaccination program with a first-generation dengue virus (DENV) vaccine, which was discontinued because of safety concerns.
A recent study assessed the relative risk of developing virologically confirmed dengue among children who did or did not receive a single dose of the Dengvaxia® (CYD-TDV) vaccine by previous DENV infections at baseline classified as none, one, and two or more infections.
This study published by The Lancet Infectious Diseases on March 22, 2024, concluded that a single dose of the Dengvaxia vaccine was ineffective in protecting against DENV among patients who had no prior history of infection or had only one prior infection.
One dose conferred significant protection against hospital admission for virologically confirmed dengue among participants who had two or more previous DENV infections at baseline during the first three years (70%, 95% CI 20–88; p=0·017) and the entire follow-up period (67%, 19–87; p=0·016).
However, young patients exposed to two or more prior DENV infections showed a significant decrease in the risk of DENV infection after receiving the first Dengvaxia dose. This protection continued for up to three years after the vaccination.
Since the study assessed the effect of only a single dose, this study's findings cannot inform public health officers' decisions on vaccination. However, the findings have implications for children who receive an incomplete vaccination regimen, and should prompt more detailed analyses in future trials on dengue vaccines.
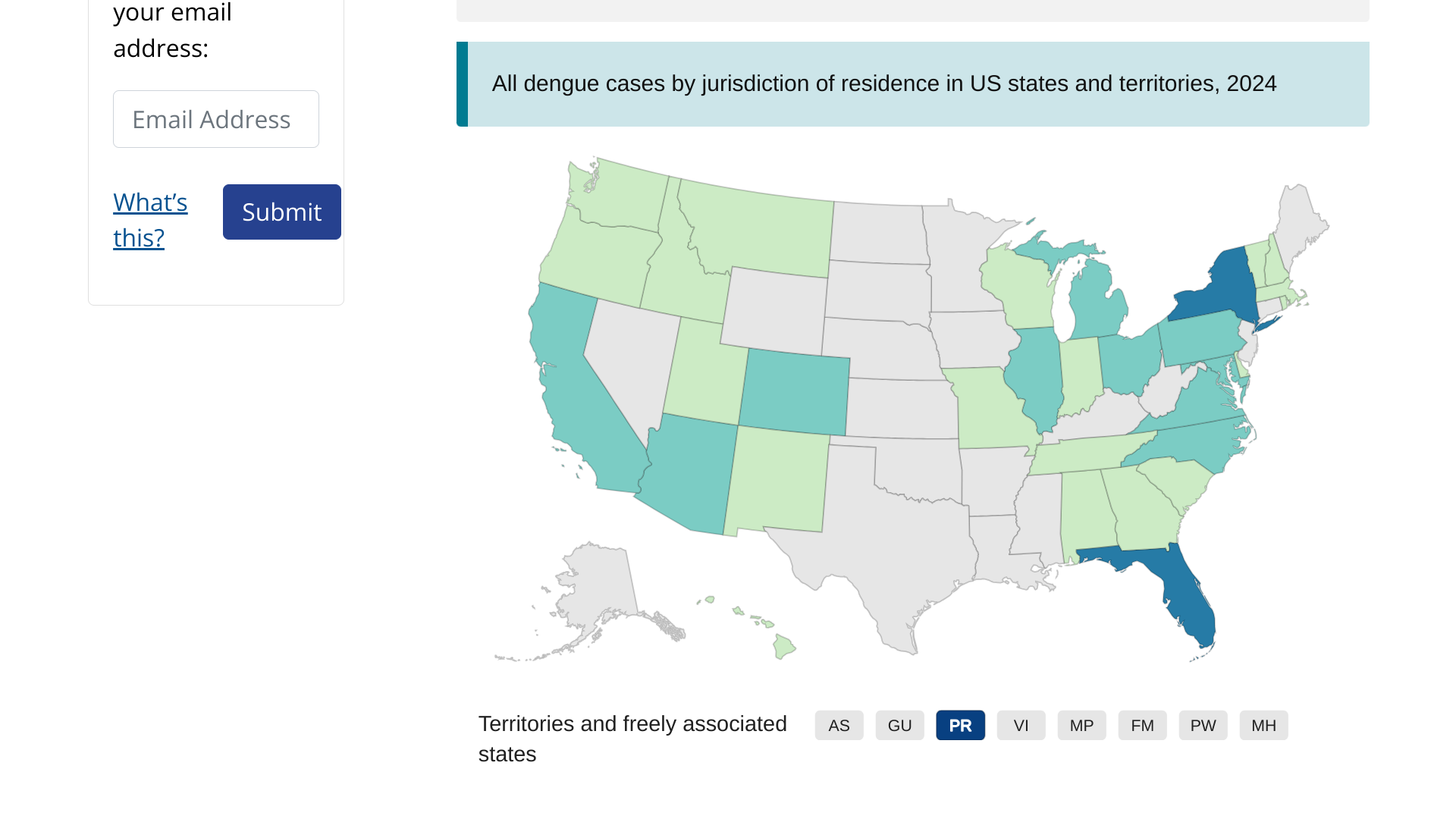
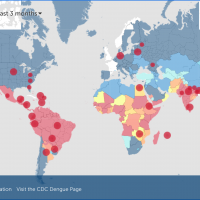
Dengue outbreaks are a global health problem in 2024.
As of March 2024, the CDC has reported over two million dengue cases worldwide, with over 500 deaths. The U.S. CDC has issued a global alert regarding dengue outbreaks in various countries.
A person infected via a mosquito bite will have no symptoms or show clinical manifestations ranging from dengue fever, a mild flu-like syndrome, to dengue shock syndrome, a life-threatening condition.
The CDC recommends speaking with a healthcare provider before visiting dengue-endemic areas like Puerto Rico to discuss vaccination and treatment options.