Search API
Uvax Bio, LLC today announced the interim analysis results from its first Phase 1 clinical trial evaluating the Company’s HIV-1 vaccine candidates, UVAX-1107 and UVAX-1197.
In the first stage of this trial, the subjects received either UVAX-1107 adjuvanted with CpG 1018® and aluminum hydroxide or placebo.
UVAX-1107 was immunogenic and generated robust IgG responses to the vaccine antigen derived from an HIV-1 strain known as BG505. 100% of subjects in the vaccine group demonstrated antibody responses after two priming vaccinations with UVAX-1107.
Antibody response titers increased >200-fold 14 days after the 2nd dose compared to the same period following the first dose.
“We are pleased that our first Phase 1 trial is progressing smoothly, and we have preliminary confirmation that UVAX-1107 was well tolerated at all doses by the study participants, and no vaccine-related serious adverse events were reported,” said Pedro Garbes, M.D., Vice President and Global Clinical Lead of Uvax Bio, in a press release on November 19, 2024.
“As per the time of this 1st interim analysis, no participant was withdrawn from the study due to local/systemic reactogenicity; local and systemic adverse events were mild to moderate, transient, and resolved on average within two days. These preliminary safety results are aligned with expectations for an adjuvanted protein-based vaccine.”
UVAX-1107 utilizes Uvax Bio’s 1c-SApNP® vaccine development platform to generate virus-like particles that closely resemble the target virus in size, shape, and multivalent antigen display; in this case, 20 copies of the native-like, prefusion-stabilized trimeric HIV-1 antigen.

As part of the Immunisation Agenda 2030, a World Health Organization (WHO) study published today in eBioMedicine named 17 pathogens that regularly cause diseases in communities as top priorities for new vaccine development.
In five out of six WHO regions, annual child deaths and contribution to antimicrobial resistance were the most heavily weighted criteria.
“Too often global decisions on new vaccines have been solely driven by return on investment, rather than by the number of lives that could be saved in the most vulnerable communities,” said Dr Kate O’Brien, Director of the Immunization, Vaccines and Biologicals Department at WHO, in a press release on November 5, 2024.
Pathogens where vaccines are approaching regulatory approval, policy recommendation, or introduction
- Dengue virus
- Group B Streptococcus
- Extra-intestinal pathogenic E. coli
- Mycobacterium tuberculosis (TB)
- RSV
Pathogens where vaccine research is needed
- Group A streptococcus
- Hepatitis C virus
- HIV-1
- Klebsiella pneumoniae
Pathogens where vaccines need to be further developed
- Cytomegalovirus
- Influenza virus (broadly protective vaccine)
- Leishmania species
- Non-typhoidal Salmonella
- Norovirus
- Plasmodium falciparum (malaria)
- Shigella species
- Staphylococcus aureus
This global prioritization exercise for endemic pathogens complements the WHO R&D blueprint for epidemics, identifying priority pathogens that could cause future epidemics or pandemics.

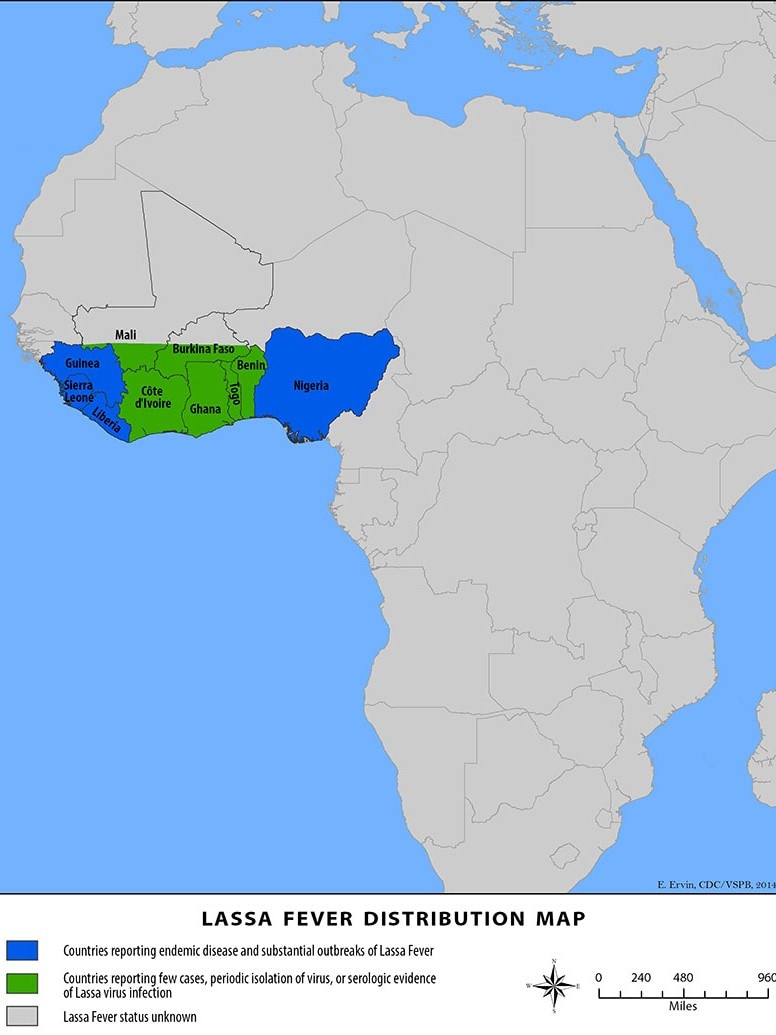
The International AIDS Vaccine Initiative (IAVI) recently announced that clinical trial sites in the Lassa fever-endemic countries of Ghana, Liberia, and Nigeria were vaccinating volunteers in IAVI's C105 study of a Lassa fever vaccine candidate.
This study is designed to evaluate the vaccine candidate’s safety, tolerability, and immunogenicity at two different dosage levels in adults, including people living with HIV, as well as in adolescents and children two years of age and older.
The IAVI C105 study results are expected in 2025. Should the vaccine candidate be found safe and efficacious, IAVI is committed to making its Lassa vaccine affordable and accessible to all needy populations.
As of August 28, 2024, no Lassa fever vaccine currently exists. However, several vaccine candidates are conducting research.
Lassa virus (LASV) is a zoonotic disease that causes the acute viral hemorrhagic illness called Lassa fever, for which treatment is limited.
People can get Lassa fever by contacting infected rats or their saliva, urine, or droppings. The U.S. CDC says that LASV can spread among people.
About 300,000 people fall ill across West Africa annually, though the actual disease burden is thought to be much higher. For these reasons, Lassa fever is featured in the World Health Organization’s R&D Blueprint and requires urgent action due to its potential to cause an outbreak of international concern.
The results of the PrEPVacc HIV vaccine trial conducted in Africa between 2020 and 2024 show conclusively that neither of the two experimental vaccine regimens tested reduced HIV infections among the study population.
The PrEPVacc vaccine trial results, announced at AIDS 2024 in Germany in July 2024, report more infections in the two vaccine arms than in the placebo arms. Still, the researchers say they cannot draw a definitive conclusion about this because the statistical ‘confidence intervals’ for the comparison are so wide, indicating high uncertainty.
PrEPVacc tested two different combinations of HIV vaccines.
One regimen combined a DNA vaccine (DNA-HIV-PT123) with a protein vaccine (AIDSVAX B/E), and the other combined the same DNA vaccine, a modified non-dividing virus vector (MVA-CMDR), and a protein-based vaccine (CN54gp140).
The vaccination schedule included four vaccine injection visits: three over approximately six months and a fourth a year after enrolment.
The PrEPVacc trial was stopped in November 2023 when it became clear to independent experts monitoring the study data that there was little or no chance of the vaccines demonstrating efficacy in preventing HIV acquisition.
Dr Peter Gilbert, Principal Investigator, who is independent of the PrEPVacc study and has no ties with it, commented in a press release on July 23, 2024, “Given the PrEPVacc results that the estimated rates of HIV-1 acquisition were higher in the vaccine arms than the placebo arm, it is important to thoroughly quantify and communicate the precision available for drawing inferences about whether the vaccines truly elevated risk or, alternatively, a statistical fluke occurred and the vaccines were indeed safe."
"P-values are incomplete tools for this task because they cannot be interpreted in terms of the question, ‘What is the chance the vaccine elevated the acquisition rate?’
“To fill this gap, I conducted a Bayesian analysis that provides answers to this desired interpretation, using the same method that I previously applied to other HIV vaccine efficacy trials."
"The result was that, for each vaccine, there is close to a 50-50 chance that the vaccine elevated acquisition risk vs. the vaccine was safe, as a synthesis of results over multiple ways to do the analysis, most importantly considering different prior distributions for vaccine efficacy," added Dr. Gilbert.
As of August 2, 2024, the U.S. FDA has approved an HIV vaccine.

Gilead Sciences, Inc. today announced full efficacy and safety results from its pivotal HIV-1 Phase 3 clinical trial.
Detailed data from the trial’s interim analysis announced in June 2024 showed that lenacapavir, the company’s twice-yearly injectable HIV-1 capsid inhibitor, demonstrated zero infections, 100% efficacy, and superiority to background HIV incidence for the investigational use of HIV prevention in cisgender women.
Lenacapavir also demonstrated superior prevention of HIV infections when compared with once-daily oral Truvada.
The new data provide details on the efficacy, safety, and tolerability of twice-yearly lenacapavir injections; drug adherence among trial participants, including poor levels of adherence to daily oral pre-exposure prophylaxis (PrEP) and high levels of adherence to lenacapavir; and demographic and behavioral characteristics of trial participants, including pregnant women and adolescents.
The data were published today in The New England Journal of Medicine.
“These stellar results show that twice-yearly lenacapavir for PrEP, if approved, could offer a highly effective, tolerable and discreet choice that could potentially improve PrEP uptake and persistence, helping us to reduce HIV in cisgender women globally,” said Linda-Gail Bekker, MBChB, DTM&H, DCH, FCP(SA), PhD, Director of the Desmond Tutu HIV Center at the University of Cape Town, South Africa, and former President of the International AIDS Society, in a press release on July 24, 2024.
“PURPOSE 1 also sets a new standard for person-centered HIV prevention trials, demonstrating what can happen when a thoughtful scientific and community-focused trial design, a promising drug candidate, and an inclusive trial implementation plan come together.”
Gilead expects results in late 2024/early 2025 from the program’s other pivotal trial, PURPOSE 2, which is assessing twice-yearly lenacapavir for PrEP among men.
Currently, there are no cures for HIV or AIDS or preventive vaccines available.

The World Health Organization (WHO) Disease Outbreak News confirmed the mpox outbreak in South Africa has expanded.
The sudden appearance of unlinked mpox cases in South Africa without a history of international travel, the high HIV prevalence among confirmed cases, and the high case-fatality ratio suggest that community transmission of the mpox virus is underway
As of July 9, 2024, 20 confirmed mpox cases, with three related fatalities in Gauteng, Western Cape, and KwaZulu-Natalhave provinces, have been reported since May 2024.
These mpox cases are South Africa's first since 2022, when five cases were reported, none fatal.
The WHO stated, 'Discussions are underway regarding options for vaccine procurement.'
Currently, two mpox vaccines are being deployed in other African countries.

A vaccine candidate for control of the cytomegalovirus (“CMV”) in patients undergoing liver transplantation dosed its initial patient in a multi-center, placebo-controlled, randomized Phase 2 clinical study.
Initially developed by the City of Hope, Triplex was exclusively licensed to Helocyte.
Triplex is a universal (non-HLA-restricted) recombinant Modified Vaccinia Ankara viral vector vaccine engineered to induce a robust and durable virus-specific T cell response to three immuno-dominant proteins [UL83 (pp65), UL123 (IE1), UL122 (IE2)] linked to CMV complications in the post-transplant setting.
The trial is funded by a grant from the U.S.S NIH’s National Institute of Allergy and Infectious Diseases to the University of Washington Seattle. This grant has provided $9 million to date, with an estimated additional $12 million over the next four years in support of the Phase 2 clinical trial.
Ajit Limaye, M.D., Professor of Medicine and Director of the Solid Organ Transplant Infectious Disease Program at the University of Washington and Principal Investigator of the “CMV vaccine in Orthotopic Liver Transplant” trial, said in a press release on May 14, 2024, “There remains a significant unmet medical need to develop new therapies that can reduce the frequency and severity of CMV events in the organ transplant setting, where CMV continues to present life-threatening complications that directly impact patient outcomes and survival.”
According to the U.S. CDC, CMV is a common virus for people of all ages.
In the U.S., nearly one in three children is already infected with CMV by age five. About 1 out of 200 babies are born with congenital CMV.
And over half of adults have been infected with CMV by age 40.
Once CMV is in a person’s body, it stays there for life and can reactivate. According to the CDC, most people with CMV infection have no symptoms and aren’t aware that they have been infected.
Helocyte is a clinical-stage company developing novel immunotherapies to prevent and treat cancer and infectious diseases, including CMV and HIV.