Search API
West Africa continues to bear the scars of the Ebola outbreak that occurred a decade ago. From 2014 to 2016, Sierra Leone, Guinea, and Liberia faced the largest Ebola outbreak since the virus was first discovered in 1976.
This Ebola outbreak resulted in over 28,000 cases and 11,000 related fatalities.
As of April 2025, more than 30 Ebolavirus disease outbreaks have been reported.
The good news is that vaccines against Ebolavirus, the pathogen responsible for those outbreaks, have since been licensed, and stockpiles have been developed.
"All Ebola outbreaks that have occurred since we had a stockpile were quickly stopped – thanks to the vaccines and rapid other response measures," says Allyson Russell, an epidemiologist and senior programme manager in Gavi's High Impact Outbreaks team, in a GAVI news article published on April 15, 2025.
However, these Ebola vaccines aren't effective against the other three orthoebolaviruses that cause severe disease, including the Sudan virus outbreak in the Republic of Uganda.
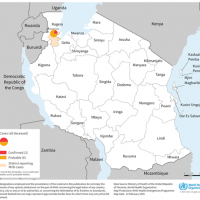
Uganda's Ministry of Health declared its eighth Sudan Ebolavirus outbreak in January 2025. Since then, about 14 cases, including four related fatalities, have been reported.
To alert the international community, the U.S. government recently updated a Level 2 Travel Health Advisory.
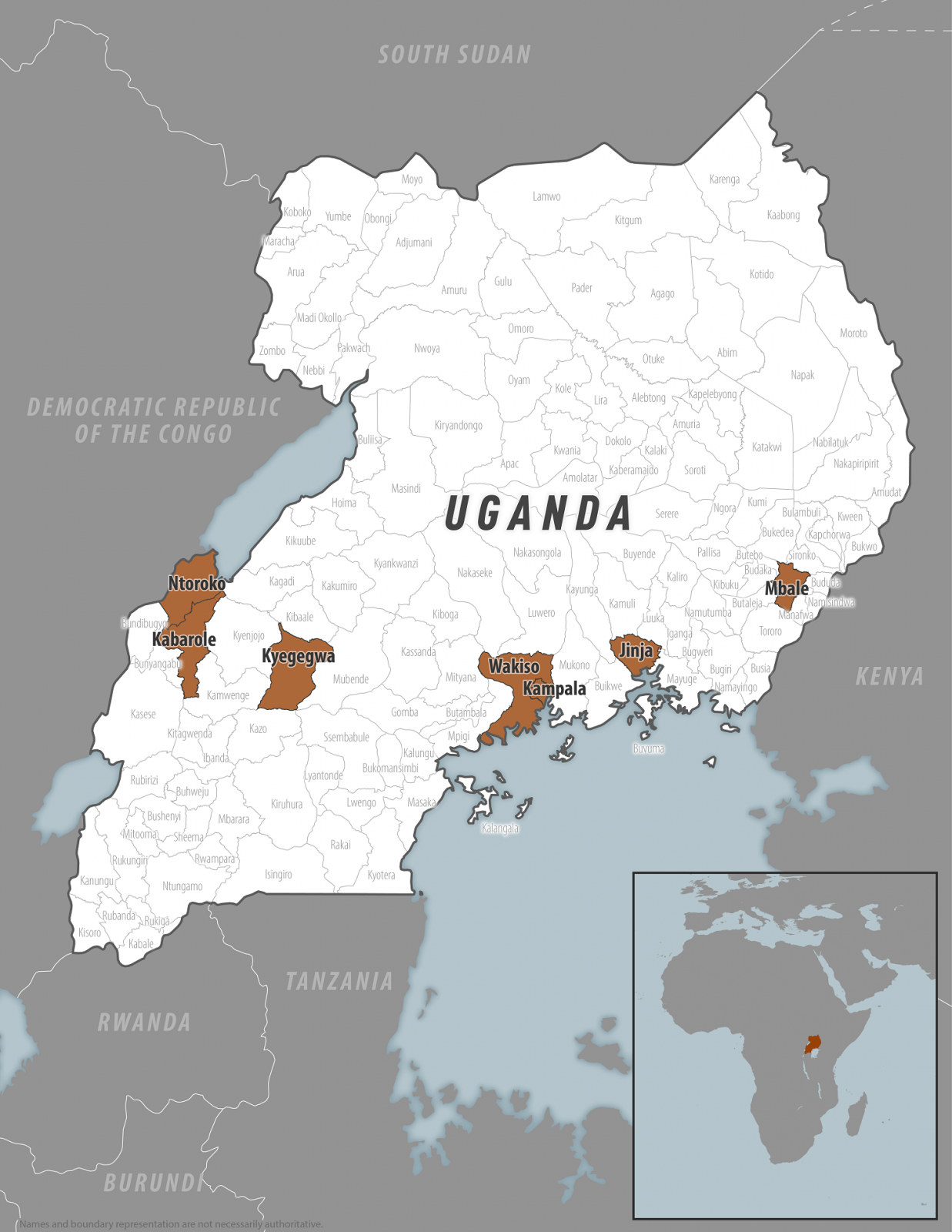
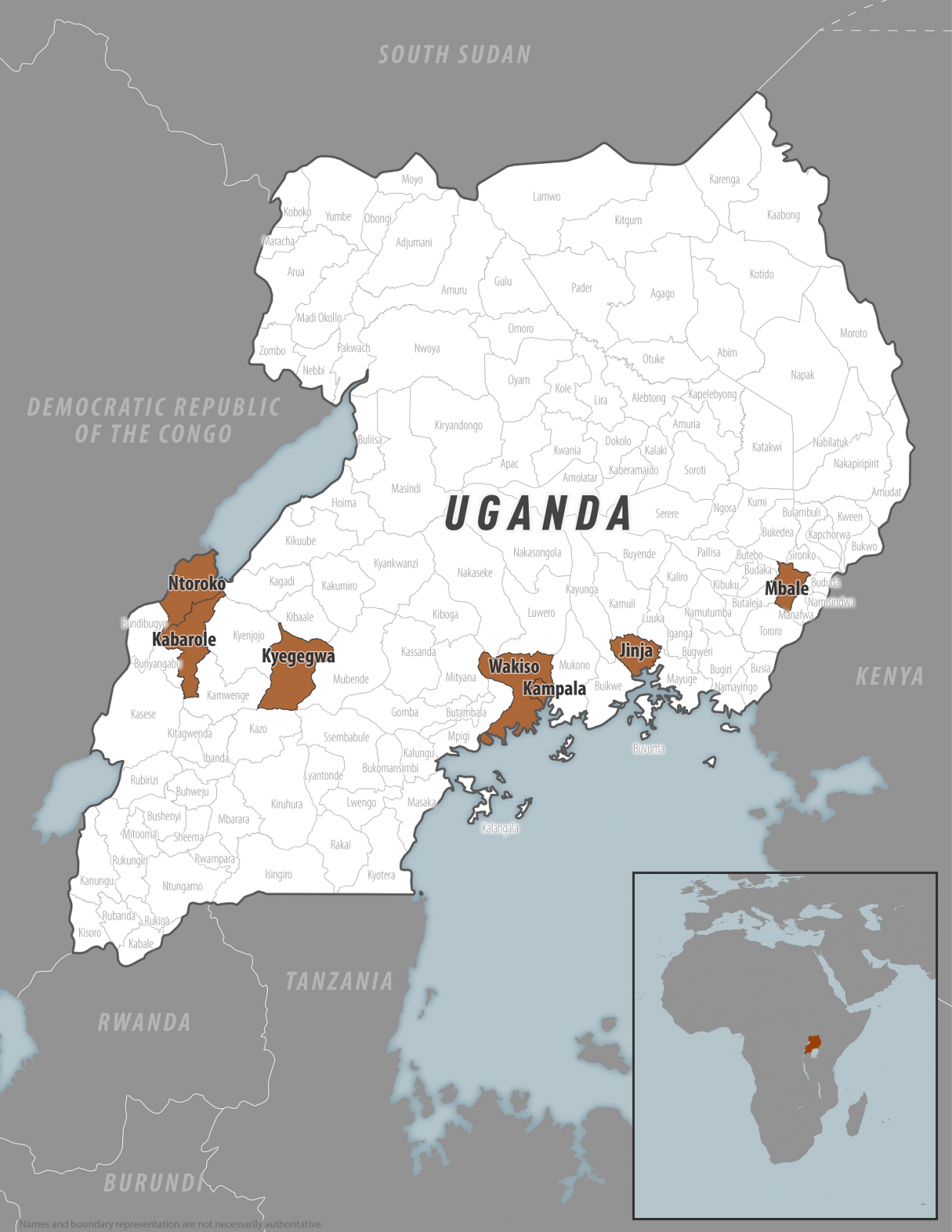
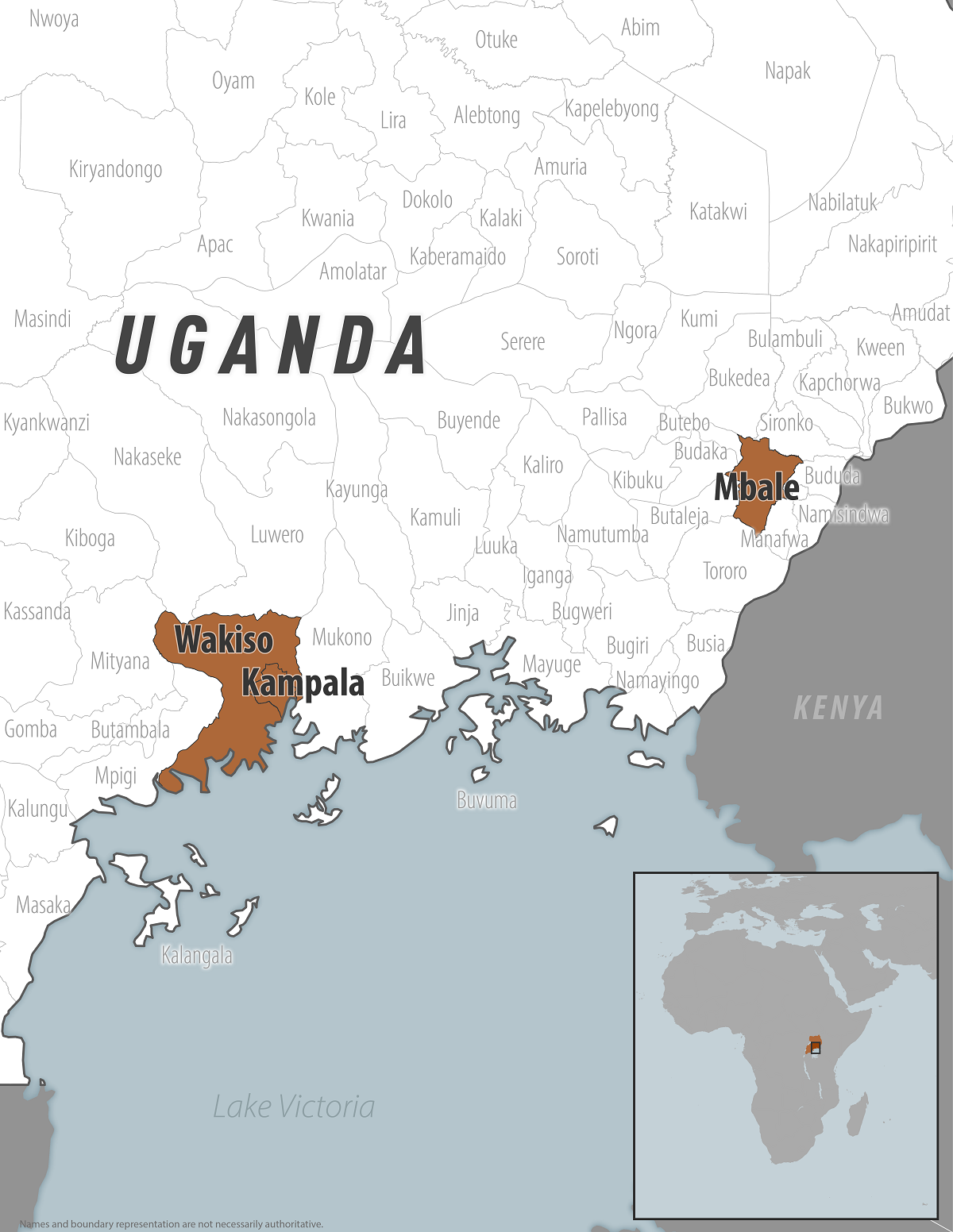
On March 12, 2025, the Centers for Disease Control and Prevention confirmed a Sudan virus outbreak in the districts of Kampala, Wakiso, Jinja, Mbale, Kyegegwa, Kabarole, and Ntoroko.
Still, the unpredictability and rapid progression of Ebola outbreaks make testing vaccine efficacy challenging.
As of April 19, 2025, no vaccines have been approved to protect people against the Sudan Ebola virus. However, candidate vaccines are being tested in the Solidarity Against Ebola human clinical studies in Africa.
Nigeria’s Ministry of Health announced today that it has received over 1,000,000 doses of the pentavalent meningococcal conjugate vaccine (Men5CV) to combat the meningococcus C and W outbreak in northern Nigeria.
This initial shipment will launch a crucial response campaign targeting individuals aged 1 to 29, the most affected demographic. The campaign will begin in Kebbi and Sokoto States and expand to Yobe State as more vaccine doses arrive.
As of April 4, 2025, this meningococcal outbreak has claimed more than 70 lives and affected over 800 individuals across 23 states.
In Africa's 'meningitis belt', seasonal outbreaks are common during the dry season, peaking between March and April due to low humidity and high dust levels.
According to the U.S. CDC, disease rates during epidemics can reach 1% of the local population.
Dr. Muhammad Ali Pate, Nigeria’s Coordinating Minister of Health and Social Welfare, stated in a press release, “The arrival of the Men5CV vaccines is a significant milestone in our fight against the meningitis outbreak."
Gavi, the Vaccine Alliance, funds the global stockpiles of vaccines against cholera, Ebola, meningitis, and yellow fever, accessible to all countries worldwide.
When visiting Nigeria in 2025, the CDC recommends routine and travel vaccines to prevent polio, measles, yellow fever, and diphtheria infections.

In response to the ongoing Sudan Ebola virus outbreak in the Republic of Uganda, the U.S. government recently updated a Level 2 Travel Health Advisory for this rare and deadly disease.
On March 12, 2025, the Centers for Disease Control and Prevention (CDC) confirmed a Sudan virus disease (SVD) outbreak in the Kampala, Wakiso, Jinja, Mbale, Kyegegwa, Kabarole, and Ntoroko districts.
Since late January 2025, about 14 cases, including four related fatalities, have been reported.
The CDC says local health authorities in Uganda are working to identify infected people and transmission sources, conduct investigations, take action to prevent further transmission, and educate communities and the public about the risks and dangers of SVD.
If you travel to Uganda, the CDC published an extensive list of activities to avoid.
About 200,000 people visited Uganda in 2024. Traveler screening at Ugandan entry points remains active as of March 2025.
Unlike Zaire Ebola, which has approved vaccines and therapeutics, SVD vaccines and therapeutics remain under development as of mid-March 2025.
From a safety perspective, the U.S. Department of State recently updated its Level 3 Travel Advisory to reflect current information.
The State Department advises reconsidering travel to Uganda and exercising increased caution due to potential risks and the unpredictable nature of public demonstrations. The U.S. Embassy in Kampala is available to support U.S. citizens.
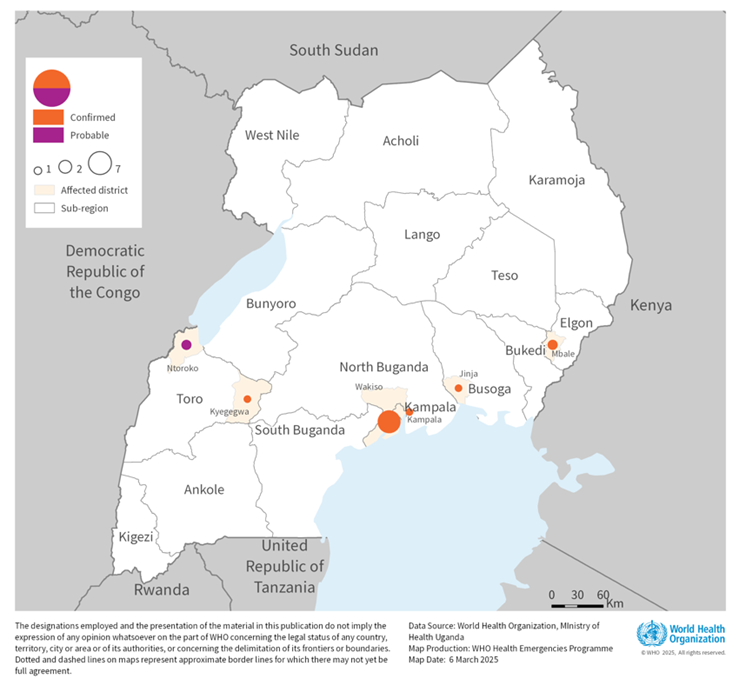
Since the recent Sudan virus disease (SUDV) outbreak was declared in the Republic of Uganda in late January 2025, a total of 14 cases, including four related fatalities, a case fatality ratio of 29%, have been reported.
As of March 5, 2025, 192 new contacts have been identified and are under follow-up in Kampala, Ntoroko, and Wakiso.
SUDV was first identified in Sudan in June 1976. This is the eighth outbreak, five in Uganda and three in Sudan.
According to the WHO's Disease Outbreak News (558) published on March 8, 2025, the Ministry of Health (MOH) stated, 'the risk of potential serious public health impact is high.' The MoH has scaled up its case management strategy to ensure sufficient capacities to provide care for all suspected and confirmed cases in all hot spots
The WHO says SVDV is a severe disease belonging to the same family as Ebola virus disease (EVD).
While several promising candidate therapeutics are currently advancing through clinical development, no licensed treatment is yet available to effectively address potential future outbreaks of EVD caused by the Sudan virus species.
A range of candidate SUDV vaccines and therapeutics are under development.
Since 2020, one vaccine and two candidate therapeutics (a monoclonal antibody and an antiviral) have been recommended. They are available in Uganda and are being assessed through randomized clinical trial protocols.
Two vaccines licensed against Zaire EVD will not provide cross-protection against SUDV.
Currently, the WHO advises against travel and/or trade restrictions to Uganda.
However, the U.S. CDC has issued a Travel Health Advisory, Level 2, for Unganda in February 2025.
The CDC says visitors to Uganda should avoid contact with sick people who have symptoms, such as fever, muscle pain, and rash, or contact with blood and other body fluids and semen from men who have recovered from EVD until testing shows that the virus is no longer in the semen.
Following the declaration of the Sudan Ebolavirus (SVD) outbreak in late January 2025, the Republic of Unganda's Ministry of Health recently reported an additional positive case in Mulago hospital who passed away on February 25, 2025.
As of early March 2025, there have been ten confirmed cases, including two related fatalities. Out of the ten confirmed SVD cases, five are health workers, and four are family members of the index case.
Additionally, nearly 300 close contacts were identified.
The World Health Organization recently wrote, 'The confirmation of a new SVD case in Kampala highlights the risk of undetected transmission, particularly given the delayed diagnosis and the child's movement across multiple healthcare facilities.'
The United Nations in Uganda has also launched an appeal to raise funds for the comprehensive three-month (March- May) response plan, seeking $11.2 million.
Future interventions will focus on seven very high-risk districts, with potential expansion to other districts based on the needs and resources in alignment with the national response plan.
"The goal is to rapidly contain the outbreak and address its impact on public health as well as associated social-economic life of affected people, in full solidarity with the Government and people of Uganda," said WHO Representative Dr. Kasonde Mwinga in a press release on March 3, 2025.
Uganda has experienced five previous SVD outbreaks.
Additionally, traveler screening at 13 Ugandan entry points remains active, with 25,364 travelers screened as of March 2, 2025. Ugandan passengers are also subject to virus screening when arriving in the United States.
Currently, there are approved vaccines and therapies for Zaire Ebolavirus but not for Sudan.
However, over 2,000 doses of IAVI's rVSV Sudan ebolavirus vaccine candidate (IAVI C108, rVSVΔG-SUDV-GP) were shipped to Uganda in early 2025. Theuse of these vaccines is being evaluated in a clinical trial.
In addition to an Ebola Travel Health Advisory, the U.S. CDC has issued polio and mpox alerts focused on Uganda in 2025. The CDC says that approved vaccines are available at travel clinics and pharmacies to prevent these diseases.
The World Health Organization (WHO) announced today that it will host an overview of the current outbreak of the Sudan Ebola virus disease in the Republic of Uganda, Africa.
On February 6, 2025, from 12:00 to 13:00 CET, Dr. Maria Van Kerkhove, WHO's Director of epidemic and Pandemic Preparedness and Prevention, and Dr. Anais Legand, WHO's Technical Officer for viral Hemorrhagic Fevers, will present the latest information about this new sudden disease outbreak and prevention and control measures, such as vaccinations.
On February 3, 2025, Uganda's Ministry of Health and its partners launched the first-ever clinical efficacy trial for an experimental Ebola vaccine derived from the Sudan virus species.
Since 2014, Ebolavirus vaccine technologies have included replication-deficient adenovirus vectors, replication-competent vesicular stomatitis, human parainfluenza vectors, and virus-like nanoparticle preparations. Zaire Ebolavirus vaccines have been approved by the U.S. Food and Drug Administration, the European Medicines Agency, and the WHO.
As of February 2025, no vaccines have been approved to protect people against the Sudan Ebolavirus.
Webinar participants can register for the free EPI-WIN using this Zoom link.
Currently, no suspected, probable, or confirmed Ebola cases related to this outbreak have been reported in the United States or outside of Uganda.