Search API
Sanofi Healthcare India Pvt Ltd (SHIPL) has recently received approval from authorities to distribute the IMOVAX-Polio vaccine, aiming to avoid any polio vaccine supply constraints in India.
The Hindu confirmed on April 5, 2024, that the IMOVAX-Polio vaccine will replace the discontinued trivalent Inactivated Polio Vaccine (IPV) ShanIP, which was launched in India in 2015.
Sanofi had previously announced that ShanIPV would discontinue in December 2023.
Access to polio vaccines is essential, as the U.S. CDC confirmed in 2024 that there have been outbreaks and poliovirus detections in 31 countries.
To help reduce the further spreading of polio, over one billion oral vaccine (nOPV2) doses have been administered in more than 35 countries. However, the nOPV2 vaccine is not available in the U.S. in 2024.
In the United States, IPV vaccines have been offered at clinics and pharmacies since 2000.
Furthermore, for certain people, the CDC recommends polio booster shots.
From a polio outbreak update, the Global Polio Eradication Initiative confirmed this week Afghanistan reported its first wild poliovirus type 1 (WPV1) case of 2024. Afghanistan and Pakistan are among the countries where WPV1 is endemic.
As of April 3, 2024, Pakistan had reported two WPV1 cases earlier this year.
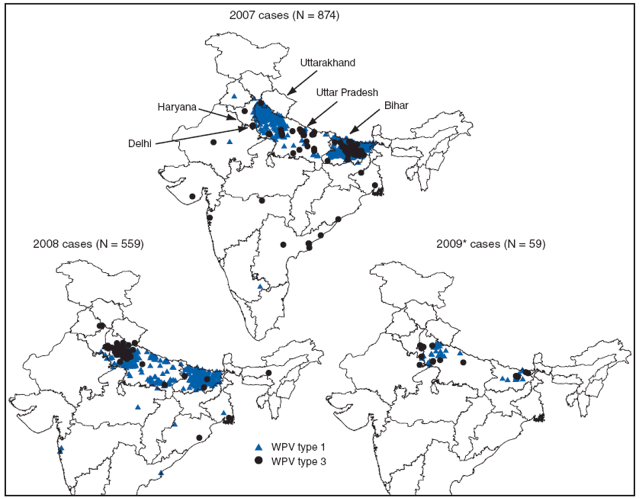
The U.S. government confirmed today that the ongoing measles outbreak in the United States is rapidly increasing.
As of April 5, 2024, the Centers for Disease Control and Prevention (CDC) reported a total of 113 measles cases had been confirmed by 18 jurisdictions: Arizona, California, Florida, Georgia, Illinois, Indiana, Louisiana, Maryland, Michigan, Minnesota, Missouri, New Jersey, New York City, New York State, Ohio, Pennsylvania, Virginia, and Washington.
Seven outbreaks (defined as three or more related cases) were reported in 2024, and 73% are outbreak-associated.
For example, in Illinois, Chicago has reported about 58 measles cases over the past weeks.
About 58% of measles cases require hospitalization.
For comparison, in 2023, 58 measles cases were reported by 20 jurisdictions, with four outbreaks.
Internationally, the CDC listed the top ten international measles outbreaks as of March 12, 2024, led by Kazakhstan, Azerbaijan, Yemen, and India.
Furthermore, the United Kingdom's multi-month measles outbreak is accelerating in April 2024.
And to alert international travelers, the U.S. CDC republished a Travel Health Notice in late March 2024, identifying measles outbreaks in 49 countries.
The CDC says measles is a vaccine-preventable disease.
Various measles vaccines are offered at clinics and pharmacies in the U.S.
According to an article written by Adam Tooze, in a world of polycrisis, in which intersecting problems compound each other and there are few easy wins, it is all the more important to recognise those policy choices that are truly obvious.
Funding vaccine development is one such investment.
Published by The Financial Times on April 1, 2024, this opinion article says modest expenditures on public health have saved tens of millions of lives, reduced morbidity, and allowed children around the world to develop into adults capable of living healthy and productive lives.
The complete, unedited article is posted at this link.

The reliable and timely detection of poliovirus cases is a critical component of polio eradication programs wrote the U.S. CDC.
Since 1988, the number of polio cases caused by wild poliovirus (WPV) has declined by 99.9%, and eradication of WPV serotypes 2 and 3 has been certified.
On April 4, 2024, a new Morbidity and Mortality Weekly Report confirmed only WPV1 continues to circulate, and transmission remains endemic in Afghanistan and Pakistan.
During 2022–2023, among 28 priority countries, 71.4% met national surveillance indicator targets, and the number of environmental surveillance sites increased.
However, the CDC says that maintaining high-quality surveillance is critical to increasing this rate and achieving the goal of global polio eradication.
To strengthen the fight against polio, health ministers from across the WHO Eastern Mediterranean Region gathered in late March 2024 for the 10th meeting of the Regional Subcommittee on Polio Eradication and Outbreaks.
"One of my key priorities as your Regional Director is to strengthen our region's public health capacities so that you have all the tools not only to end the transmission of polio but also to ensure that polio can indeed never make a comeback," commented Dr. Hanan Balkhy in a media release.
Another component of polio eradication is vaccinations.
According to the CDC, two types of polio vaccines are in use in 2024.
While the inactivated (killed) polio vaccine is offered in clinics and pharmacies in the U.S., over 1 billion novel oral polio vaccinations have recently occurred.
The nOPV2 vaccine has been offered in more than 35 countries worldwide.
The nOPV2 vaccine is being deployed under the WHO's pre-qualified Emergency Use Listing procedure, the first use for a polio vaccine.

Throughout the global measles outbreak in 2024, about 49 countries have reported cases.
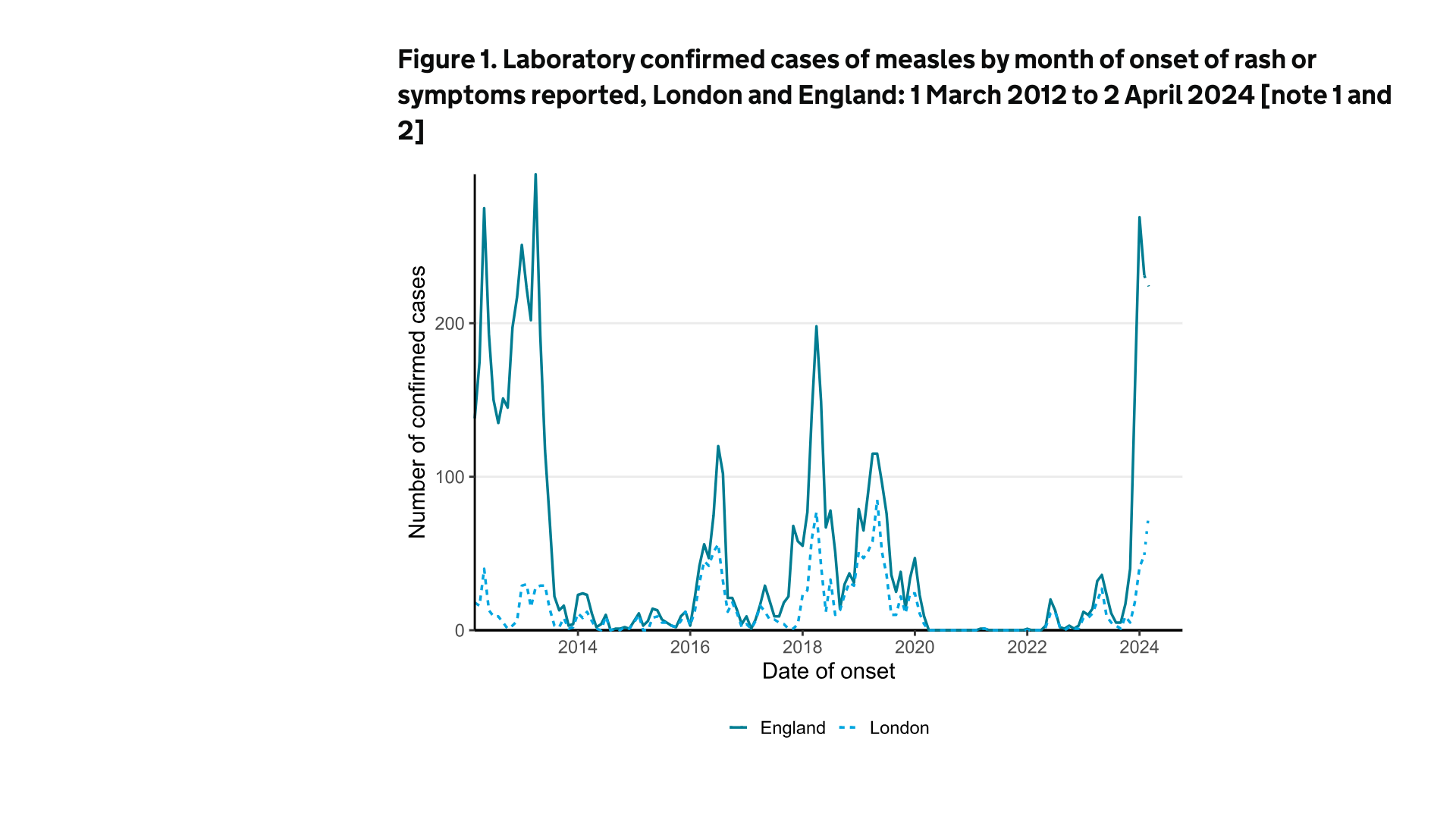
Unfortunately, the United Kingdom is rapidly becoming a leader.
On April 4, 2024, the U.K. Health Security Agency (UKHSA) reported 269 measles cases in January 2024, 231 in February, and 224 (to date) in March 2024.
In total, there have been 934 measles cases since October 2023.
About 54% of these cases have been in the West Midlands and 21% in London.
Dr. Vanessa Saliba, UKHSA Consultant Epidemiologist, offered these insights in a press release, "We are continuing to see measles cases in all regions of England, with cases particularly high in the West Midlands and London, so it is vital that two doses of the MMR vaccine fully protect people."
"It only takes one case to get into a community with low vaccination rates for measles to spread rapidly, especially in schools and nurseries."
"We know that hundreds of thousands of children around the country, particularly in some inner-city areas, are still not fully vaccinated and are at risk of serious illness or life-long complications, but measles is completely preventable with vaccination."
Worldwide, the U.S. Centers for Disease Control and Prevention listed the top ten international measles outbreaks as of March 12, 2024, led by Kazakhstan (21,740), followed by Azerbaijan, Yemen, and India.
To alert international travelers, the CDC republished a global Watch-Level 1, Practice Usual Precautions, Travel Health Notice on March 22, 2024, identifying measles outbreaks in numerous countries.
In the United States, Chicago, Illinois, continues leading the measles outbreak in 2024.
As of April 3, 2024, Illinois has reported 56 measles cases this year, including the Chicago Department of Public Health (53) measles cases.
In the U.S., measles vaccines are generally available at clinics and community pharmacies.
Morris & Dickson today announced it became the first U.S. commercial distributor of the JYNNEOS® vaccine. This second-generation vaccine is U.S. FDA-approved to prevent mpox and smallpox disease.
On April 3, 2024, Morris & Dickson confirmed receiving the first U.S. shipment of JYNNEOS vaccines.
This vaccine must be safely stored at minus 20 degrees Celsius. Morris & Dickson's state-of-the-art distribution techniques feature this storage and transport capability.
"We are proud to be a key partner in expanding access to this first-to-market vaccine," says Layne Martin, CEO of Head of Specialty at Morris & Dickson, in a press release.
"JYNNEOS meets a critical public health need and helps ensure equitable access to healthcare, which in turn helps significantly prevent the spread of mpox to at-risk populations."
As of April 2024, U.S. healthcare providers in the U.S. can order JYNNEOS to make it available for at-risk individuals at local pharmacies and physician offices in addition to public health clinics.
In 2023, the U.S. CDC confirmed that the effectiveness of the JYNNEOS vaccine against mpox ranges from 36% to 75% after one dose and 66% to 89% for two doses. As of 2024, third-dose boosters have not been clinically approved.
Founded in 1841, Morris & Dickson is now approaching $6 billion in annual sales, making it the industry's largest independently owned full-line distributor. Independent pharmacies in the U.S. rely on Morris & Dickson for quick responses and straightforward business practices.
Since the global mpox outbreak began in early May 2022, more than 32,000 cases have been reported in the U.S., representing a third of all cases reported worldwide.
Bavarian Nordic's JYNNEOS (MVA-BN®, IMVAMUNE®) vaccine is available in various countries.

As influenza vaccine producers prepare for the 2024 - 2025 flu season, innovative vaccine candidates are progressing in clinical research, focused on enhancing efficacy and safety.
CureVac N.V. today announced interim data from an ongoing Phase 2 study, which is part of the combined Phase 1/2 study of its seasonal influenza vaccine candidate.
The purpose of this clinical trial (NCT05823974) is to find and confirm the dose and asses the reactogenicity, safety, and immune response of GlaxoSmithKline's (GSK) messenger RNA (mRNA)-based multivalent seasonal influenza vaccine (GSK4382276A) candidates administered in healthy younger and older adults.
Results from the planned interim analysis showed that the multivalent vaccine candidate using CureVac's proprietary second-generation mRNA backbone boosted antibody titers against all encoded flu strains and across all age groups and tested dose levels, including the lowest tested dose.
"The Phase 2 interim data show that CureVac's highly effective and flexible mRNA technology platform puts us on the right track to advance our joint seasonal influenza vaccine program," said Dr. Myriam Mendila, Chief Development Officer of CureVac, in a press release on April 4, 2024.
"Results regarding influenza A strains were strong. Immunogenicity for B strains was also in line with our expectations in view of other initial mRNA-based clinical flu development programs."
"We are confident that planned optimizations will improve performance against these historically challenging influenza strains."
The multivalent candidate was selected from a comprehensive Phase 1 part, which tested vaccine candidates with up to eight separate mRNA constructs per candidate.
It was designed for broad antigen coverage, encoding antigens that matched all World Health Organization (WHO) recommended flu strains.
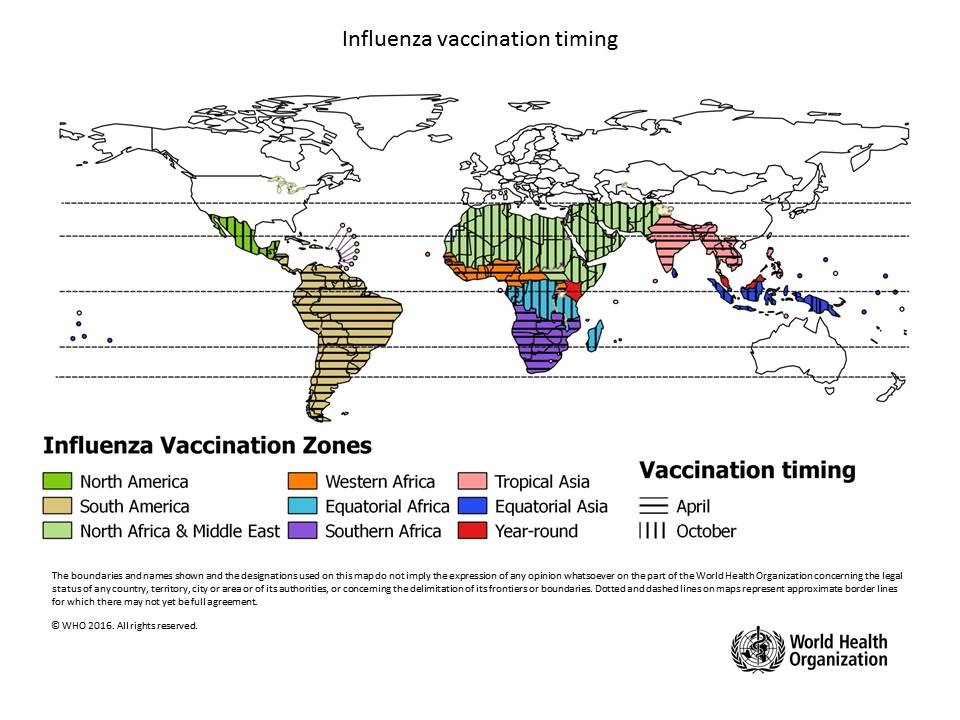
The WHO says flu shot campaigns should be timed according to local conditions.
Countries are encouraged to analyze local surveillance information to assess their seasonality pattern at both national and subnational levels, as appropriate, to make evidence-based decisions on the timing of vaccination campaigns.
An advanced vaccine candidate for the Lassa fever virus (LASV) today announced the start of its second phase of clinical trials.
This is a significant development, as no approved vaccines for LASV are currently available.
The trial sponsor, International AIDS Vaccine Initiative (IAVI), a nonprofit scientific research organization, confirmed in a press release on April 4, 2024, that participants at HJF Medical Research International in Nigeria had been vaccinated in the first Phase 2 clinical trial of the vaccine candidate rVSV∆G-LASV-GPC.
The IAVI C105/PREVAIL15 study began on March 4, 2024, and is expected to enroll over 600 people in Nigeria, Ghana, and Liberia, with results expected in 2025.
As of March 2024, 27 states in Nigeria have reported at least one confirmed case of LASV.
The vaccine has been developing since 2018 and has been supported and funded by CEPI and the European & Developing Countries Clinical Trials Partnership.
According to IAVI, rVSV∆G-LASV-GPC uses the same recombinant vesicular stomatitis virus vector platform as ERVEBO®, the single-dose Zaire ebolavirus vaccine licensed in North America, Europe, and various African countries.
“Continued outbreaks of Lassa fever and the emergence of Ebola Sudan in Uganda both underscore the need to have vaccines for known disease threats available for evaluation and use during outbreak situations – the overarching goal of IAVI’s emerging infectious disease program,” stated Swati Gupta, DrPH, MPH, vice president and head of emerging infectious diseases and epidemiology, IAVI.
The virus causes acute viral hemorrhagic illness and results in approximately 5,000 deaths and 300,000 illnesses in West Africa each year.
Furthermore, LASV has been included in the World Health Organization's R&D Blueprint of priority pathogens for which accelerated research and development and countermeasures are urgently needed.
In addition to rVSV∆G-LASV-GPC, several other LASV vaccine candidates are conducting clinical research in 2024.