Search API
Research findings presented at the European Society of Clinical Microbiology and Infectious Diseases Global Congress show that a lower dose of the JYNNEOS® (MVA-BN) mpox vaccine is safe and generates a six-week antibody response equivalent to the standard regimen.
The results announced by the U.S. NIH on April 27, 2024, suggest that antibody responses contributed to the effectiveness of dose-sparing mpox vaccine regimens used during the 2022 U.S. outbreak.
The authors noted that because no defined correlates of protection against mpox—immune processes confirmed to prevent disease—these findings cannot predict the efficacy of dose-sparing regimens with certainty.
Real-world data from the U.S. Centers for Disease Control and Prevention and others have shown similar vaccine effectiveness for the dose-sparing regimen given intradermally and the standard regimen given subcutaneously.
According to the NIH, a study of the standard JYNNEOS regimen in adolescents is ongoing and will report findings later this year.
An earlier press release stated that the antibodies produced by JYNNEOS against mpox wane significantly within a year of receiving the vaccination. In contrast, among individuals who had received childhood smallpox vaccination, most had detectable VACV IgG one year after vaccinations.
In April 20224, JYNNEOS became commercially available in the U.S. by establishing additional pathways for vaccine procurement, distribution, and reimbursement by public and private payers, including community pharmacies.
Mpox vaccinations are essential since the number of mpox cases in the U.S. has more than doubled compared to Week #15 in 2023. As of April 13, 2024, 750 mpox cases had been reported, compared to 336 cases at the same time last year.

The Global Polio Eradication Initiative (GPEI) today announced the Kingdom of Saudi Arabia has pledged $500 million over the next five years to support the elimination of polio.
The GPEI stated on April 28, 2024, that these funds will yearly protect more than 370 million children with polio vaccines.
It will also facilitate the delivery of other life-saving interventions, such as nutritional supplements and bed nets, to underserved communities and strengthen health systems to better prepare countries for emerging health threats.
“For decades, polio inflicted lifelong suffering on children and families. Today, we take another step toward finally eradicating it, thanks to the generous contributions of the Kingdom of Saudi Arabia. When we invest in eradicating polio, we make immunization and health systems more resilient, we equip nations to better respond to public health challenges, and most importantly, we ensure that more children can live healthy lives,” said Bill Gates in a press release.
The announcement builds on a long history of support from Saudi Arabia and regional partners to the global polio eradication effort as well as across the Eastern Mediterranean – the last region where wild polio is endemic.
For more than two decades, Saudi Arabia has not only financially supported the GPEI but has also advocated for polio eradication and access to other life-saving vaccines in the Eastern Mediterranean and beyond.
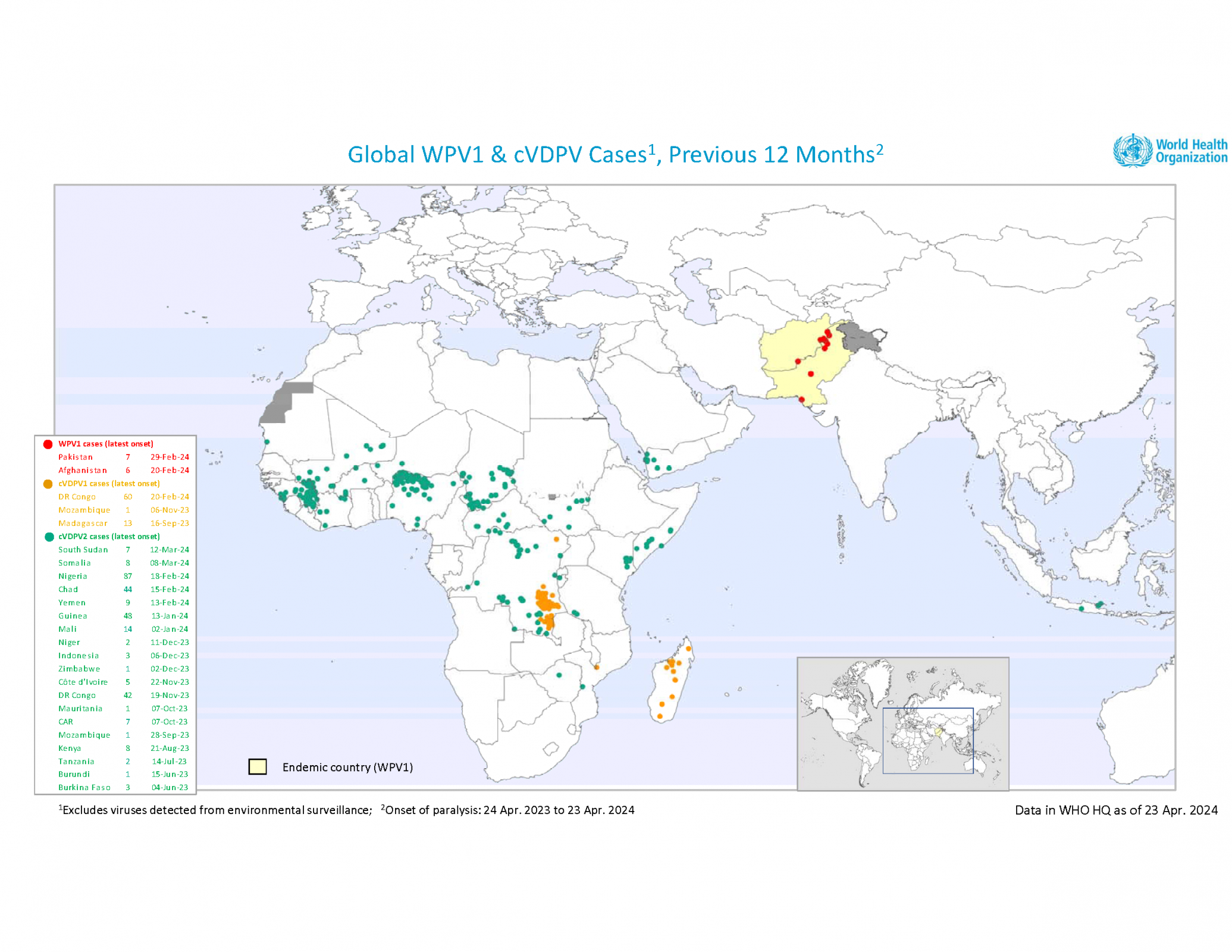
Unfortunately, the GPEI also reported several polio detections this past week.
- Afghanistan: 10 WPV1-positive environmental samples
- Pakistan: two WPV1-positive environmental samples
- Angola: one cVDPV2-positive environmental sample
- Nigeria: two cVDPV2 cases
- Somalia: one cVDPV2 case
- South Sudan: three cVDPV2 cases
- Yemen: one cVDPV2 case and eight positive environmental samples
- Zimbabwe: one cVDPV2-positive environmental sample
To better address the evolving risk of type 2 variant poliovirus (cVDPV2), the next-generation novel oral polio vaccine type 2 (nOPV2) is being deployed in countries affected by these outbreaks.
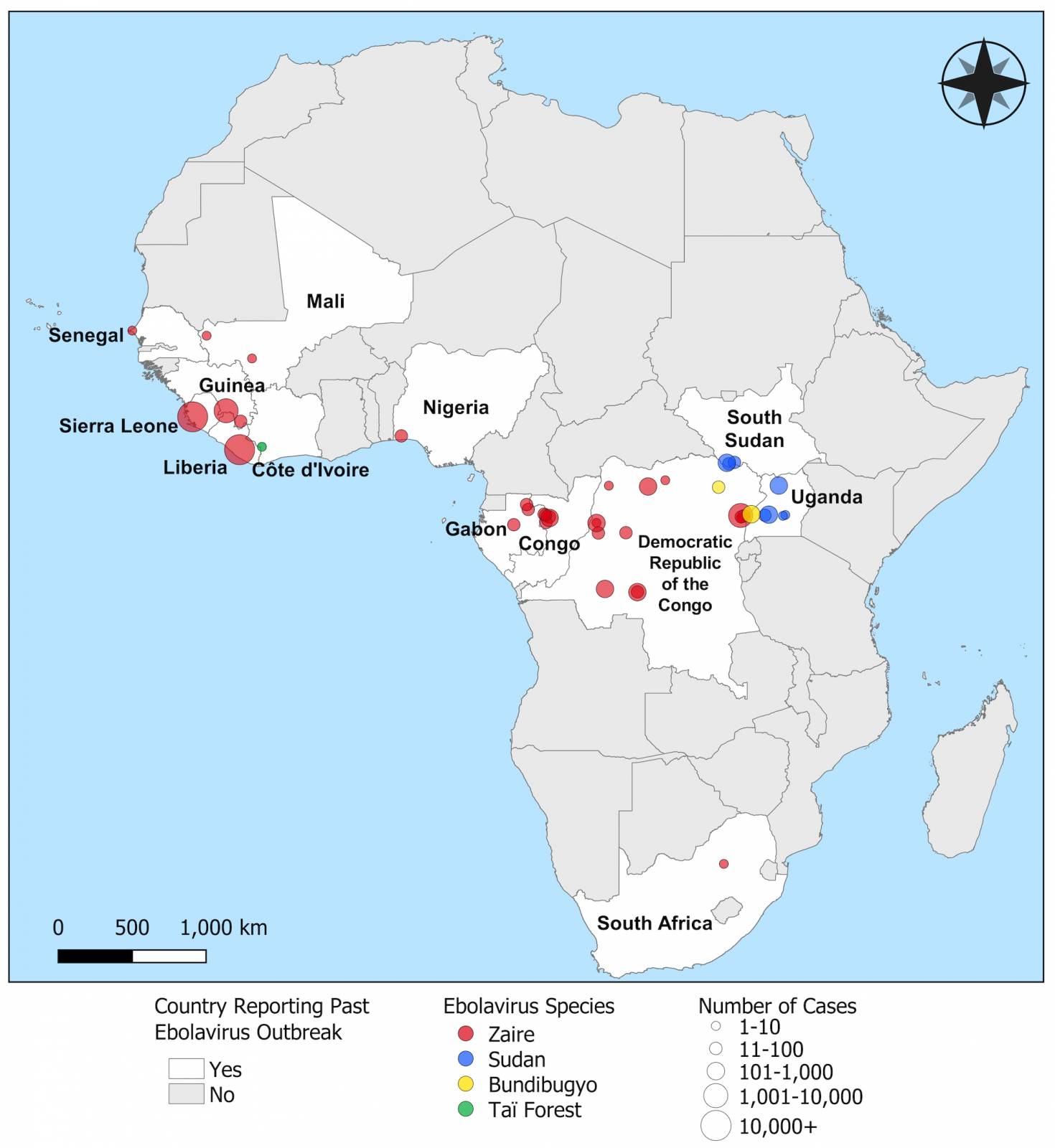
A new U.S. Centers for Disease Control and Prevention (CDC) report describes the use of Ebola vaccines and the role of the stockpile developed and managed by the International Coordinating Group (ICG) on Vaccine Provision.
A total of 145,690 doses have been shipped from the ICG stockpile since 2021.
Because Ebola outbreaks since 2021 have been limited, most doses (139,120; 95%) shipped from the ICG stockpile have been repurposed for preventive vaccination of high-risk groups, compared with 6,570 (5%) used for outbreak response.
The CDC wrote on April 25, 2024, repurposing doses for preventive vaccination could be prioritized in the absence of Ebola outbreaks to prevent transmission and maximize the cost-efficiency and benefits of the stockpile.
Currently, two licensed vaccines are recommended for the prevention of Ebola caused by Orthoebolavirus zairense:
- The 1-dose rVSVΔG-ZEBOV-GP (ERVEBO®) was licensed by the European Medicines Agency and the Food and Drug Administration in 2019 and is indicated for use in persons aged >12 months. The vaccine has also been approved in Burundi, Central African Republic, Côte d’Ivoire, Democratic Republic of the Congo (DRC), Ghana, Guinea, Republic of the Congo, Rwanda, Sierra Leone, Uganda, and Zambia. The U.S. FDA has approved ERVEBO.
- The 2-dose Ad26.ZEBOV and MVA-BN-Filo (Zabdeno/Mvabea) regimen is recommended for preventive vaccination in areas at lower risk for Ebola (or areas neighboring an outbreak) because the complete regimen is administered over 56 days.
To effectively manage the global Ebola stockpile, the International Coordinating Group on Vaccine Provision ensures equitable and timely access to vaccine doses for Ebola outbreaks, says the CDC.
Ebolaviruses were first discovered in 1976 near the Ebola River in what is now the Democratic Republic of Congo. According to the CDC, viral and epidemiologic data suggest that ebolaviruses existed long before the initial recorded outbreaks occurred.
Pfizer Inc. recently announced positive top-line data from the ongoing pivotal Phase 3 clinical trial evaluating a single dose of ABRYSVO versus placebo in adults 18 to 59 years of age at risk of developing severe respiratory syncytial virus (RSV)-associated lower respiratory tract disease (LRTD).
Highlights from this study include participants' achieving at least a fourfold increase in serum neutralizing titers for RSV-A and RSV-B one month following receipt of ABRYSVO compared to pre-vaccination.
During the trial, ABRYSVO was well-tolerated, and safety findings were consistent with those from previous investigations of ABRYSVO in other populations.
Among these U.S. adults, 9.5% have a chronic condition that puts them at risk of severe RSV disease, and this percentage rises to 24.3% among persons 50 to 64 years of age.
However, no RSV vaccines have been approved for adults aged 18 to 59.
Annaliesa Anderson, Ph.D., Senior Vice President and Head, Vaccine Research and Development, Pfizer, stated in a press release on April 9, 2024, "We are excited to address a significant unmet need, pending regulatory authority approval, as ABRYSVO has the potential to become the first and only RSV vaccine for adults 18 years and older."
In May 2023, the FDA approved ABRYSVO for the prevention of LRTD caused by RSV in individuals 60 years of age or older.
Pfizer is currently the only company that has an approved RSV vaccine for protecting older adults and infants through maternal immunization.
The respiratory syncytial virus causes RSV disease. There are two major subgroups of RSV: RSV-A and RSV-B. Both subgroups cause disease and can co-circulate or alternate predominance from season to season.

Today, the U.S. Centers for Disease Control and Prevention (CDC) confirmed that seasonal influenza activity continues to decline nationally and in most areas of the country.
On April 26, 2024, the CDC's FluView Key Updates for Week #16 highlighted that outpatient respiratory illness declined and is below baseline for the third week in a row. Nationally, the number of weekly flu hospital admissions has been decreasing since January.
Unfortunately, the CDC reported six additional influenza-associated pediatric deaths occurring during the 2023-2024 season were reported, increasing the flu season total to 148 pediatric deaths.
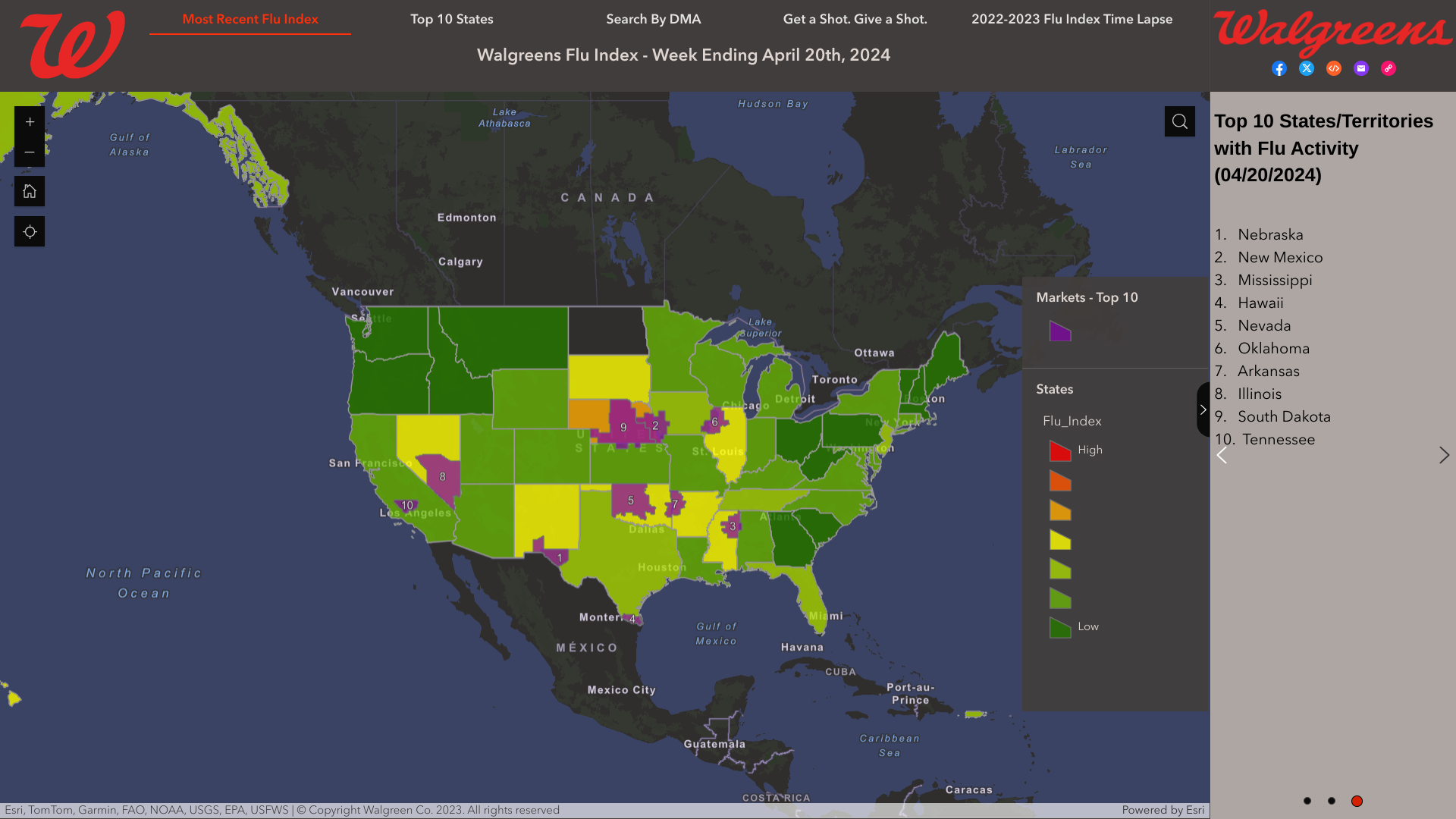
Seperately, the Walgreens Flu Index map shows local activity (04/20/2024) in these cities:
- El Paso, Texas (Las Cruces, N.M.
- Omaha, Neb.
- Columbus-Tupelo-West Point-Houston, Miss.
- Harlingen-Weslaco-Brownsville-McAllen, Texas
- Oklahoma City, Okla.
- Davenport, Iowa-Rock Island-Moline, Ill.
- Ft. Smith-Fayetteville-Springdale-Rogers, Ark.
- Las Vegas, Nev.
- Lincoln & Hastings-Kearney, Neb.
- Bakersfield, Calif.
The CDC reconfirmed there also are influenza vaccines and prescription flu antiviral drugs available at pharmacies in the U.S.