Search API
According to researchers at the University of Minnesota, a common diabetes drug has been found to reduce the amount of SARS-CoV-2 coronavirus and lower the risk of COVID-19 rebound symptoms when administered early during non-severe illness.
"The results of the study are important because COVID-19 continues to cause illness, both during acute infection and for months after infection," said Carolyn Bramante, MD, principal investigator and an assistant professor at the U of M Medical School, in a press release on May 2, 2024.
This study was published in the journal Clinical Infectious Diseases on May 1, 2024.
These researchers wrote that in this randomized, placebo-controlled phase 3 clinical trial of outpatient treatment of SARS-CoV-2, metformin significantly reduced the viral load.
The mean SARS-CoV-2 viral load was reduced 3.6-fold with metformin relative to placebo (−0.56 log10 copies/mL; 95% confidence interval [CI], −1.05 to −.06; P = .027).
Those who received metformin were less likely to have a detectable viral load than placebo at day five or day 10 (odds ratio [OR], 0.72; 95% CI, .55 to .94).
Viral rebound, defined as a higher viral load on day ten than on day 5, was less frequent with metformin (3.28%) than with placebo (5.95%; OR, 0.68; 95% CI, .36 to 1.29).
The metformin effect was consistent across subgroups and increased over time.
In a related commentary, the study authors wrote, "This study makes a strong case for a potential effect of metformin on COVID-19.... and prompts a reevaluation of existing data in support of its use."
The Rainwater Charitable Foundation, The Parsemus Foundation, UnitedHealth Group, and Fast Grants funded the clinical trial. Drs. Bramante and Jacinda Nicklas' time was supported by the National Institutes of Health

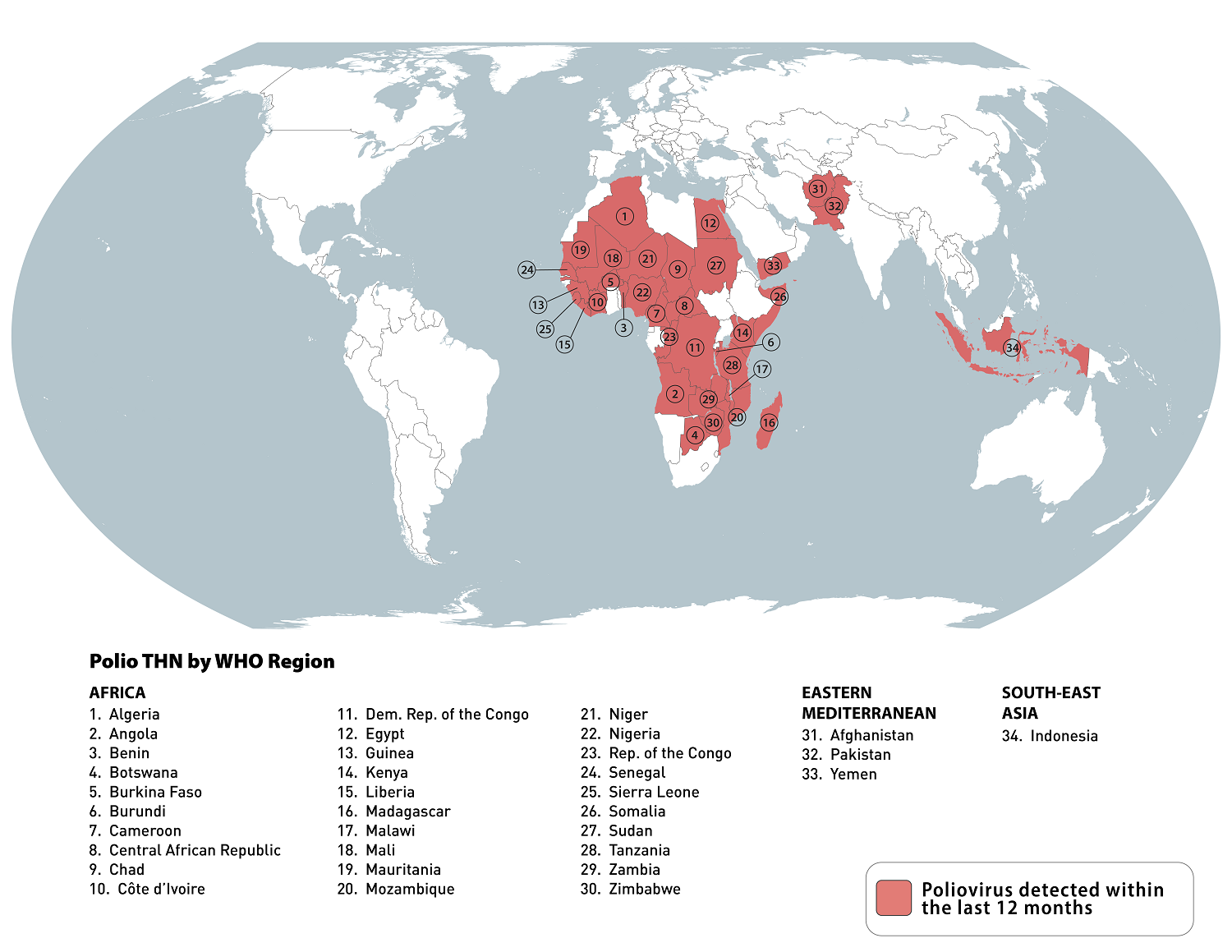
According to an updated Travel Health Advisory, there are now 34 destinations that may have circulating poliovirus.
As of May 3, 2024, the Global Polio Eradication Initiative confirmed various countries reported WPV1, cVDPV2, and cVDPV1 polio cases this year.
On April 26, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reissued its Level 2 - Practice Enhanced Precautions notice regarding recent polio outbreaks.
The CDC says that adults who previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of polio vaccine before traveling to any destination listed. And to ensure that anyone who is unvaccinated or incompletely vaccinated completes the routine polio vaccine series before departing abroad.
In the U.S., polio vaccination services are generally available at health clinics and community pharmacies.
Previously, the World Health Organization (WHO) confirmed during the 38th meeting of the IHR Emergency Committee for Polio that the spread of the poliovirus remained a Public Health Emergency of International Concern and recommended its extension for a further three months to July 2024 to reduce the risk of the international spread of poliovirus.
Unfortunately, the U.S. is included in the WHO April 2024 notice.
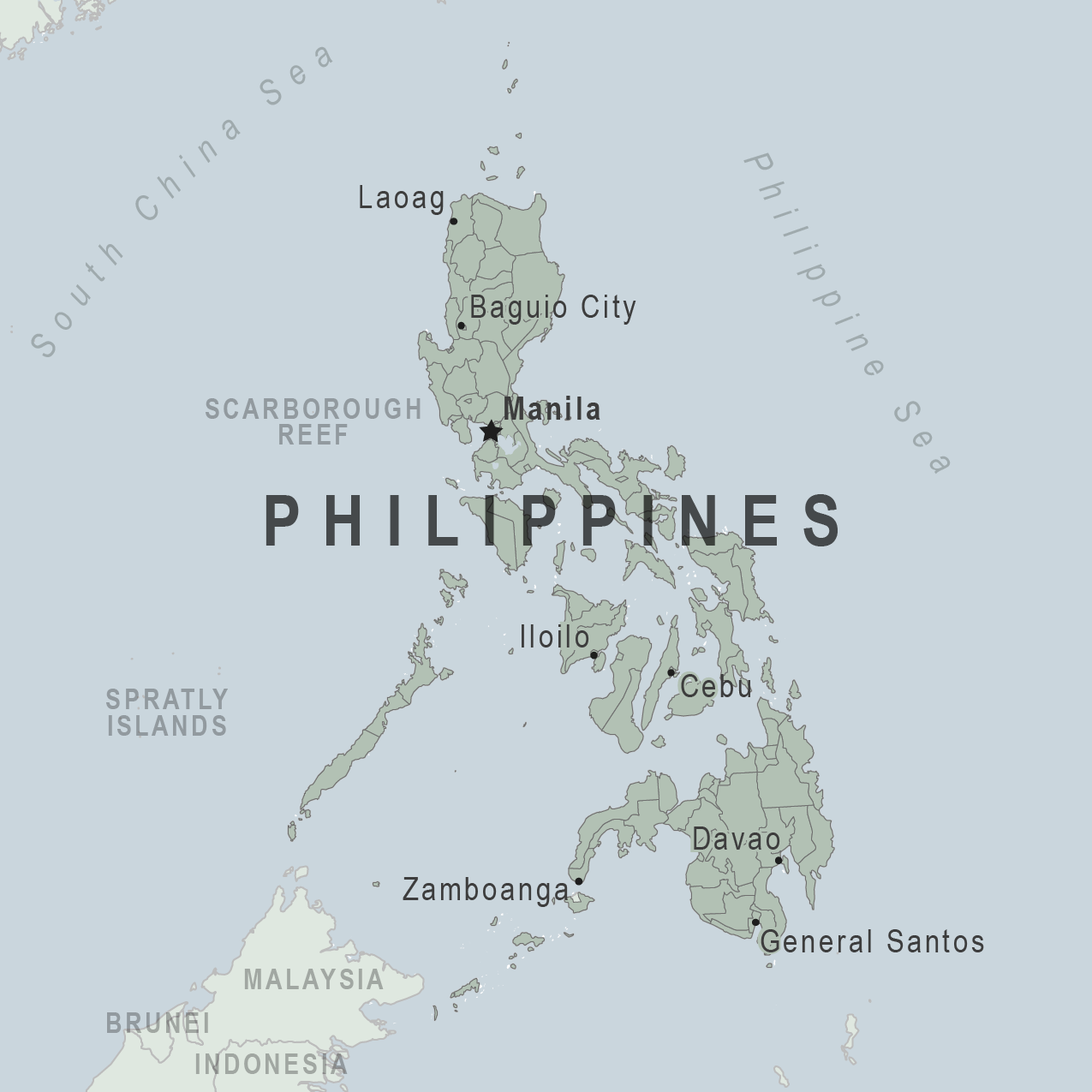
Amidst the global measles outbreak of 2024, a new Health Alert has been issued for the Republic of the Philippines.
On May 2, 2024, the U.S. Embassy in Manila announced the U.S. Centers for Disease Control and Prevention (CDC) issued a Level 1 Travel Health Notice for Measles in the Philippines.
The CDC recommends that all travelers to the Philippines, including infants and pre-school-aged children, be fully vaccinated against measles.
If you are unsure whether you or your travel companions are fully protected against measles, schedule an appointment to visit a healthcare provider at least one month before traveling abroad so that you have enough time to be fully immunized, says the CDC.
If you don't think you can safely receive a measles-containing vaccine, talk to your doctor and consider making alternative travel plans.
In the U.S., measles vaccines are generally available at retail pharmacies.
Visit the CDC Travelers Health webpage for travelers to the Philippines to learn more.
As of late April 2024, the CDC listed 51 countries confronting measles cases this year.
This list includes Afghanistan, Angola, Armenia, Azerbaijan, Benin, Burkina Faso, Burundi, Cameroon, Central African Republic, Chad, Côte d'd'IvoireIvory Coast), Democratic Republic of the Congo, Djibouti, Equatorial Guinea, Ethiopia, Gabon, Ghana, India, Indonesia, Kazakhstan, Kyrgyzstan, Lebanon, Liberia, Libya, Malaysia, Mauritania, Nepal, Niger, Nigeria, Pakistan, Philippines, Qatar, Republic of South Sudan, Republic of the Congo, Romania, Russia, Senegal, Somalia, Sri Lanka, Sudan, Syria, Tajikistan, Timor-Leste (East Timor), Togo, Turkey, United Arab Emirates, Uzbekistan, Yemen, Zambia.
Moderna, Inc. today reported financial results and provided business updates for the first quarter of 2024.
On May 2, 2024, the Company reported $167 million in Spikevax® (COVID-19 vaccine) sales in the first quarter of 2024, compared to $1.9 billion in the same period in 2023, a decrease of 91%.
This sales decline aligns with the anticipated transition to a seasonal COVID-19 vaccine market.
"As we anticipate the launches of our Spikevax 2024-2025 formula and RSV vaccine, we are exercising financial discipline and have intensified our focus on building and utilizing AI technologies to further streamline operations and enhance productivity," said Stéphane Bancel, Chief Executive Officer of Moderna, in a press release.
"With ten late-stage programs and additional new programs advancing toward pivotal studies, we continue to expect numerous product milestones this year across our vaccines and therapeutics portfolio."
"This is the start of a banner year for our vaccine platform as we continue to advance mRNA medicines for patients. This is just the beginning."
A recent announcement of positive interim results from the NEXTCove Phase 3 trial showed that Moderna's next-generation COVID-19 vaccine (mRNA-1283) elicited a higher immune response against both the Omicron BA.4/BA.5 and original virus strains of SARS-CoV-2 compared to Moderna's licensed COVID-19 vaccine (mRNA-1273.222).
The next-generation vaccine is designed to be refrigerator-stable and paves the way for a combination vaccine against influenza and COVID-19 (mRNA-1083). The Company is engaging with regulators on the next steps.
And Moderna's Phase 3 trial of its combination vaccine against seasonal flu and COVID-19 (mRNA-1083) is fully enrolled. The Company anticipates data from the study in 2024.

The results from a literature search published on April 26, 2024, identified 16 study records that fit the inclusion criteria for reviewing the effectiveness of the JYNNEOS® (MVA-BN) vaccine against mpox.
Where the study population was exclusively or primarily those receiving pre-exposure prophylactic vaccination, JYNNEOS's adjusted vaccine effectiveness (VE) estimates ranged from 35% to 86% (n=8 studies) for one dose.
JYNNEOS's VE for two doses ranged from 66% to 90% (n=5) for two doses.
Where only post-exposure prophylactic vaccination was assessed, adjusted VE estimates were reported for one dose only at 78% and 89% (n=2).
These researchers concluded that 'despite heterogeneity in study design, setting, and at-risk populations, the reported VE estimates against symptomatic mpox infection for one or two doses supports deployment of JYNNEOS for mpox outbreak control.
The U.S. Food and Drug Administration initially approved JYNNEOS for smallpox in September 2019 and extended its authorization for mpox in 2022. As of late 2023, about 1.2 million doses had been administered in 57 U.S. Jurisdictions.
As of May 2024, healthcare providers in the U.S. can order JYNNEOS through their preferred wholesaler and distribution partners to make it available for at-risk individuals at pharmacies.
According to a recent analysis, JYNNEOS booster (third) doses have not been recommended.
The World Health Organization recently published Mpox External Situation Report #32, which confirmed that from January 2022 through March 2024, a cumulative total of 95,226 mpox cases, including 185 deaths, were reported.
Note: Bavarian Nordic Inc., the owner of JYNNEOS, funded the research presented in this manuscript.

According to a post on X by @DrPatSoonShiong, the initial 1,000 doses of ANKTIVA® (N-803) shipped on May 1, 2024.
On April 22, 2024, ImmunityBio Inc. announced that the U.S. Food and Drug Administration (FDA) had approved ANKTIVA plus BCG vaccine therapy for treating certain patients with BCG-unresponsive bladder cancer.
The company says Anktiva is the backbone of ImmunityBio's Quantum Oncotherapeutics immunotherapy-based vaccine approach for treating multiple tumor types.
"We hypothesized that activation and proliferation of natural killer cells through IL-15 stimulation could rescue T cells after checkpoint failure, regardless of tumor type or location. As with non-muscle invasive bladder cancer, we believe that ANKTIVA enhanced the NK and T cell activity critical for targeting and killing cancer cells which have entered the phase of tumor evasion and resistance," said Patrick Soon-Shiong, M.D., Executive Chairman and Global Chief Scientific and Medical Officer at ImmunityBio, in a press release on April 25, 2024.
In 2024, Anktiva is expected to cost $35,800 per dose. ImmunityBio's Patient Assistance Program is expected to ensure access to ImmunityBio's innovative cancer treatment for those in financial support.




