Urinary Tract Infection (UTI) Clinical Trials
Urinary Tract Infection (UTI) Vaccine Clinical Trials 2025
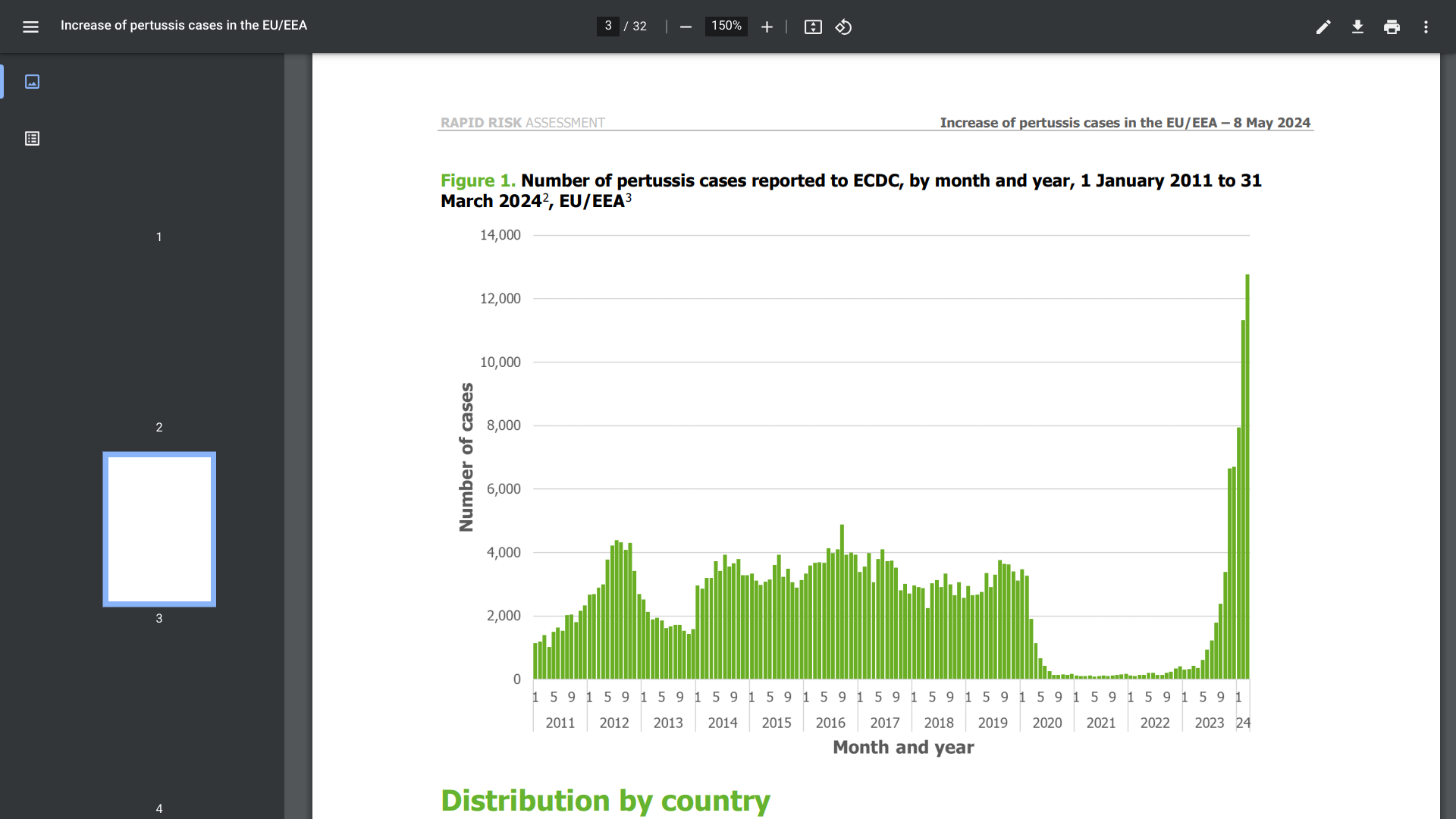
Clinical trials for a urinary tract infection (UTI) vaccine and treatment are being conducted in 2025. To register for a clinical trial, please visit this Clinical Trials Link. This GSK UTI vaccine candidate study is seeking participants in the United States, Argentina, Belgium, South Africa, and Spain.
Recurrent UTI Vaccine Information
Uromune™ (MV140) is a self-administered oral spray vaccine for recurrent urinary tract infections approved for adults in certain countries. A vaccination appointment can be requested at PassportHealth.
Urinary Tract Infection Treatments
In March 2025, the US FDA approved Blujepa (gepotidacin) for the treatment of female adults (≥40 kg) and paediatric patients (≥12 years, ≥40 kilograms) with uncomplicated urinary tract infections caused by the following susceptible microorganisms: Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii complex, Staphylococcus saprophyticus, and Enterococcus faecalis.
RECCE® 327 is an intravenous and topical therapy developed to treat serious UTI/Urosepsis infections caused by Gram-positive and Gram-negative bacteria, including their superbug forms.
UTILITY therapeutics Ltd. pivmecillinam (Pivya™, Selexid, Melysin, Coactab) tablets are approved for the treatment of female adults with uncomplicated UTIs. Pivmecillinam is expected to be available in the USA in 2025.
UTI Vaccine Appointment Requests
UTI vaccination requests can be submitted using this appointment link.