Search API
According to the U.S. Centers for Disease Control and Prevention (CDC), the amount of respiratory illness (fever plus cough or sore throat) causing people to seek healthcare is low nationally.
As of May 17, 2024, the CDC says no jurisdictions experienced moderate, high, or very high activity last week.
Furthermore, nationally, emergency department visits with diagnosed COVID-19, influenza, and RSV are at low levels.
While there are approved vaccines that help prevent these diseases, the CDC suggests speaking with a healthcare professional about the best time to be immunized.
The World Health Organization (WHO) has reported a critical shortage of Oral Cholera Vaccines (OCV), which is affecting cholera outbreak responses.
As of May 16, 2024, the WHO's External Situation Report #14 classified the global resurgence of Cholera as a grade 3 emergency, its highest internal level for emergencies, due to the increasing number of outbreaks and their geographic spread, as well as the shortage of vaccines and other resources,
Since January 2023, OCV requests have surged, with 15 countries requesting 82 million doses, nearly double the 46 million doses produced during this period.
As of early May 2024, the OCV vaccine stockpile had 3.2 million doses, below the global target of five million.
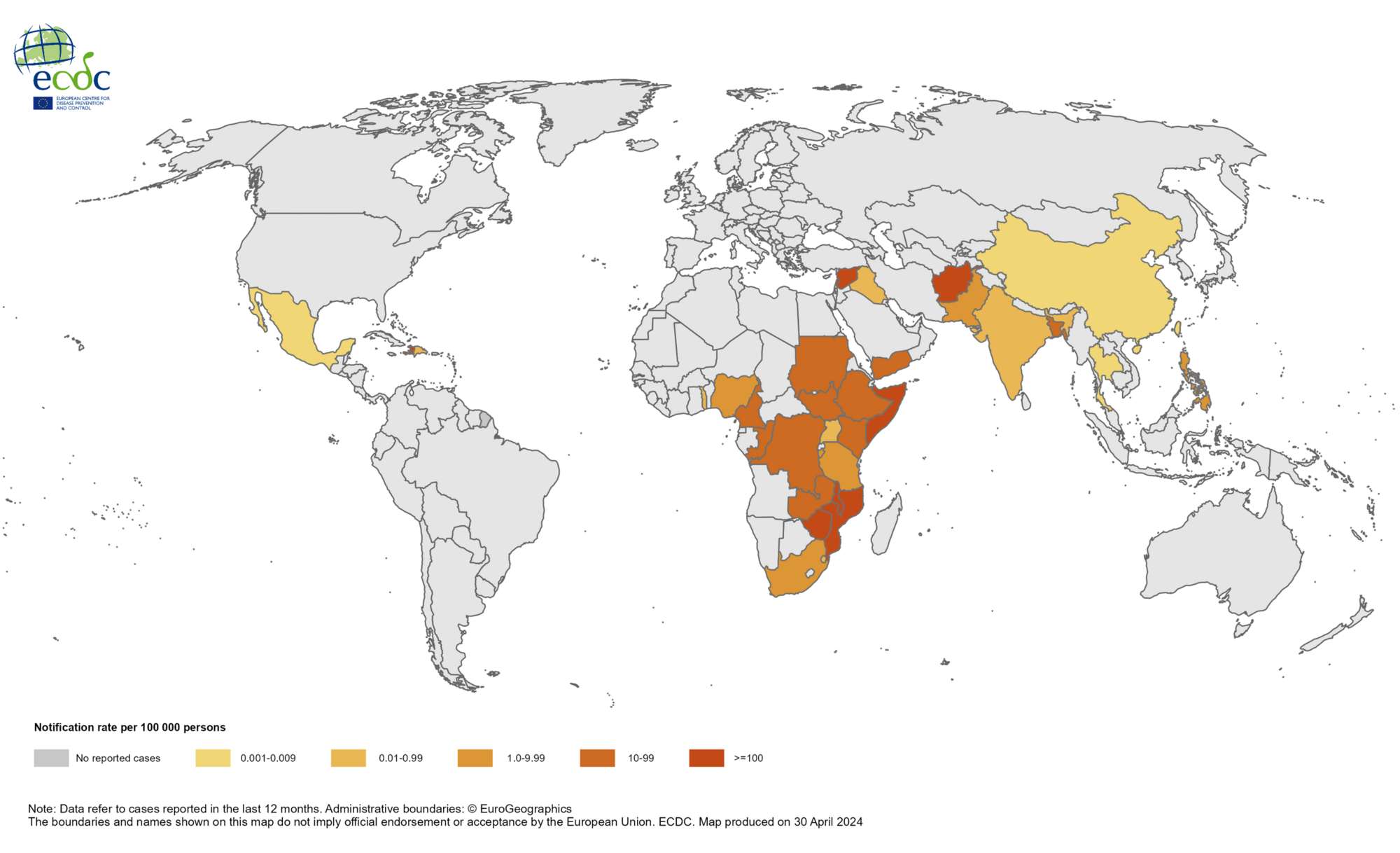
From January 2024 to April 2024, a cumulative total of 145,900 cholera cases and 1,766 deaths were reported from 24 countries across five WHO regions.
No cholera outbreaks were reported in the United States.
The U.S. CDC says Cholera is an infection of the intestines caused by the bacterium Vibrio cholerae. You can get Cholera from drinking water or eating food containing cholera bacteria.
Most people who get Cholera don't get sick. However, Cholera can cause life-threatening watery diarrhea and vomiting.
As of May 2024, there are various OCVs available.
Two doses of OCV protect against Cholera for at least three years. Since October 2022, only one dose of vaccine has been used in outbreak response, offering protection for about a year.
In the U.S., cholera vaccines are generally available at travel clinics and certified pharmacies.
During the early stages of the recent pandemic, AstraZeneca's Evusheld antibody was an effective therapeutic option. However, as the SARS-CoV coronavirus evolved, the U.S. FDA withdrew Evusheld's authorization in early 2023.
According to the company's press release on May 16, 2024, positive high-level results from the SUPERNOVA Phase III COVID-19 pre-exposure prophylaxis (prevention) trial showed sipavibart (AZD3152), an investigational long-acting antibody (LAAB), demonstrated a statistically significant reduction in the incidence of symptomatic COVID‑19 compared to control in an immunocompromised patient population.
SUPERNOVA is a large Phase III global trial providing the only efficacy data in immunocompromised patients, demonstrating the potential benefit of a COVID-19 antibody against recent SARS-CoV-2 variants.
Sipavibart was well tolerated in the trial, and preliminary analyses showed that adverse events were balanced between the control and sipavibart arms.
Iskra Reic, Executive Vice President, Vaccines and Immune Therapies, AstraZeneca, commented, "Immunocompromised patients currently have limited or no options for COVID-19 protection and continue to face a significant burden of disease, despite often being fully vaccinated."
"Sipavibart has the potential to prevent COVID-19 in the immunocompromised, and we will now work with regulatory authorities globally to bring sipavibart to these vulnerable patients."
Sipavibart is not a preventive vaccine.
It was engineered using the same antibody scaffold as Evusheld and optimized with the same half-life extension, reduced Fc effector function, and complemented the C1q binding platform.
The reduced Fc effector function aims to minimize the risk of antibody-dependent disease enhancement - a phenomenon in which virus-specific antibodies promote, rather than inhibit, infection and/or disease.
According to the press release, AstraZeneca is in dialogue with regulatory authorities on potential authorization or approval pathways.

Eisai Co., Ltd. and Biogen Inc. recently announced the initiation of a rolling submission of a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for LEQEMBI® subcutaneous autoinjector for weekly maintenance dosing.
According to the company's press release on May 14, 2024, the injection process requires less time than the intravenous (IV) formulation.
If approved by the FDA, the LEQEMBI autoinjector could be used to administer LEQEMBI at home or medical facilities.
LEQEMBI (lecanemab-irmb) is a humanized immunoglobulin gamma 1 monoclonal antibody. It is indicated for the treatment of Alzheimer's disease (AD) in patients with Mild Cognitive Impairment or mild dementia stage of disease.
LEQEMBI is now approved in the U.S., Japan, and China, and applications have been submitted for review in the European Union, Australia, Brazil, Canada, Hong Kong, Great Britain, India, Israel, Russia, Saudi Arabia, South Korea, Taiwan, Singapore and Switzerland.
Previously, Eisai submitted a Supplemental BLA for monthly LEQEMBI IV maintenance dosing to the FDA in March 2024.
According to the Alzheimer's Association's Alzheimer's Disease Facts and Figures, an estimated 6.5 million Americans aged 65 and older live with dementia due to AD.
As of May 16, 2024, the FDA has not approved a vaccine that prevents AD.

VBI Vaccines Inc. today announced a business update and financial results for the March 31, 2024 quarter. The Company's innovative Hepatitis B vaccine (PreHevbrio®, PreHevbri®) has quickly gained global market access and produced measurable revenues.
PreHevbrio is the only 3-antigen hepatitis B vaccine, comprised of the three surface antigens of the hepatitis B virus.
"To date in 2024, our focus has centered around pipeline execution, expanding access and increased uptake of PreHevbrio in targeted market segments, and execution of strategic partnerships to drive opportunity for our portfolio assets, create shareholder value, and strengthen our balance sheet," said Jeff Baxter, VBI's President and CEO, in a press release on May 16, 2024.
PreHevbrio product revenue net increased 105% from Q1 2023, with $1.0 million earned in Q1 2024. During early 2024, PreHevbrio U.S. sales continue to demonstrate substantial growth over 2023, with over 80% of the 2023 full-year volume being sold in the first five months of 2024.
Furthermore, VBI partners with Valneva SE to make PreHevbri available in certain European countries. PreHevbri was launched in the UK, Sweden, Netherlands, and Belgium in 2023, and in early 2024, PreHevbri also became available in Denmark and Norway.

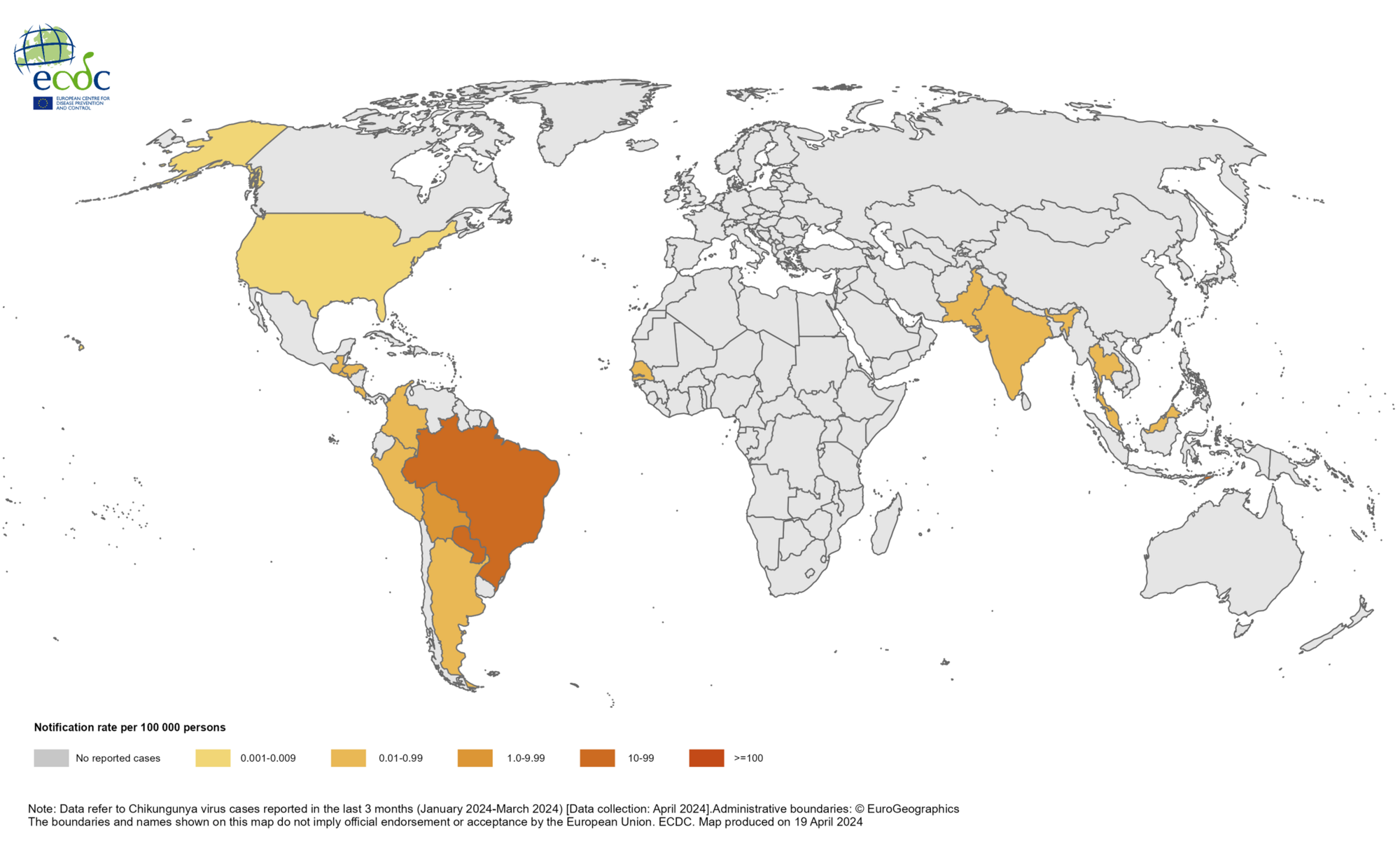
An analysis published in the journal Travel Medicine and Infectious Disease revealed that 86% of travelers diagnosed with chikungunya virus (CHIKV) infection over two years experienced joint pain, significantly impacting their quality of life.
Furthermore, 42.6% of patients with chronic arthralgia reported a recurrence of symptoms once they felt they had disappeared.
Published on May 15, 2024, this study underscores the need for comprehensive pre-travel advice and the effective management of patients enhancing outcomes.
Additionally, an in-depth knowledge of this disease is essential for early identification, particularly in non-endemic areas with competent mosquito vectors for virus transmission.
The World Health Organization says CHIKV was identified in nearly 115 countries in 2024, primarily in the Region of the Americas. In 2024, over 160,000 CHIKV cases and 50 deaths have been reported worldwide.
In the United States, the U.S. FDA-approved IXCHIQ® single-dose chikungunya vaccine is awaiting a commercial release in 2024.
The U.S. Food and Drug Administration Current and Resolved Drug Shortages and Discontinuations Report says as of May 14, 2024, Teva Pharmaceuticals USA, Inc. mefloquine hydrochloride tablet medication (Tablet, 250 mg, NDC 0555-0171-78) is in limited supply through August 2024.
The company's contact number is 800-545-8800.
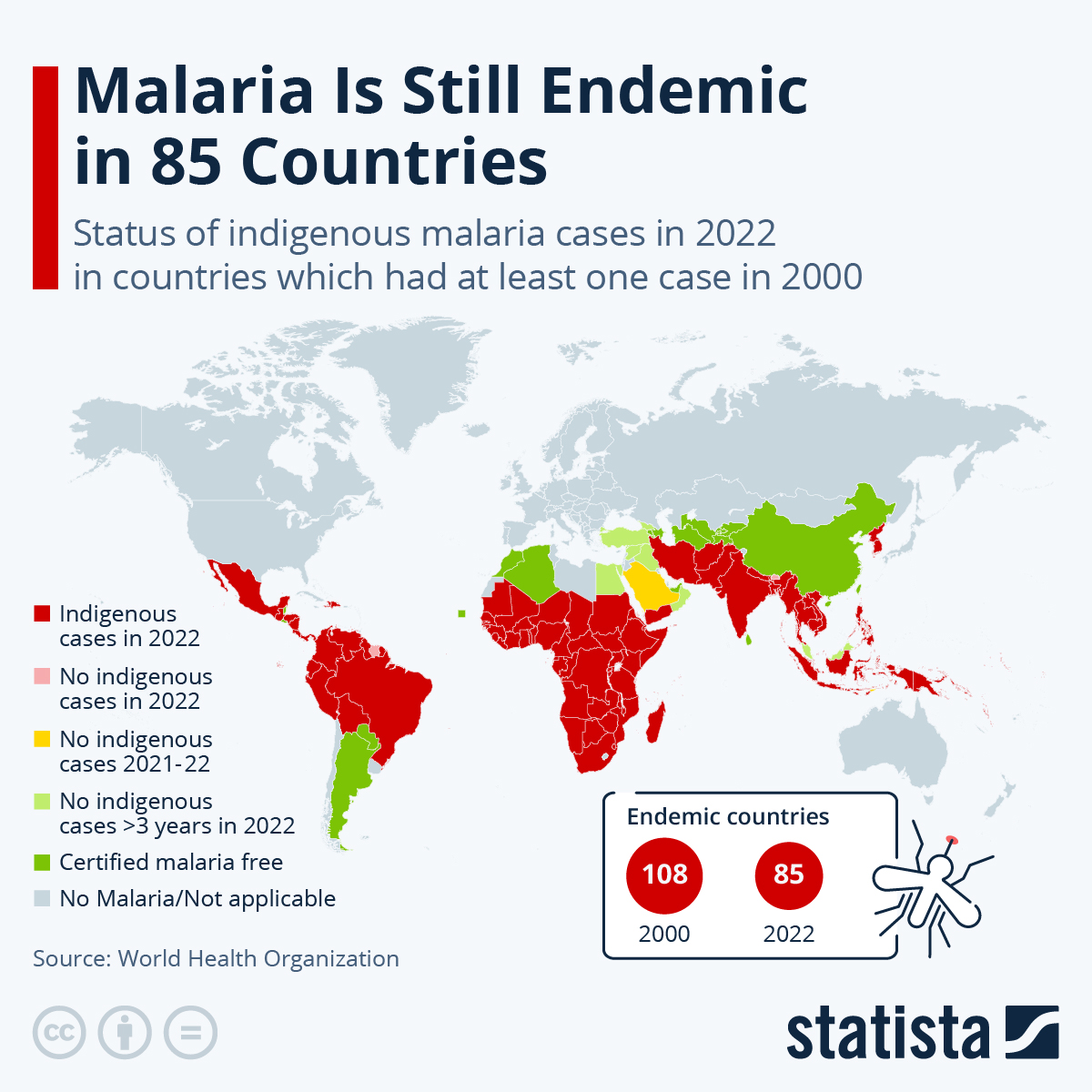
In 2024, the Pan American Health Organization estimated that approximately 41 million people are living in areas where the risk of infection by mosquito-carrying Malaria is considered moderate to high. Most malaria infections in the United States and its territories occur among persons who have traveled to regions with ongoing malaria transmission.
While Teva's product shortage may disrupt summer vacation plans, pharmacists and travel clinics may offer alternative products.
As of May 15, 2024, numerous malaria cases have been reported in the United States in 2024.
While two malaria vaccines are available in Africa, neither are offered in the United States.
Blackstone today announced the launch of the Blackstone Life Sciences portfolio company Uniquity Bio, a clinical-stage drug development company focused on immunology and inflammation.
Uniquity Bio launches with the U.S. FDA's acceptance of its Phase 2 investigational new drug application for solrikitug, a monoclonal antibody targeting TSLP.
Solrikitug is a highly potent anti-TSLP monoclonal antibody that prevents the binding of TSLP to its receptors. Given TSLP's position as the "master switch" cytokine at the top of the inflammatory cascade, solrikitug could have potential utility in various immunology and inflammation programs.
Solrikitug was in-licensed from Merck & Co., Inc.
Up to $300 million in capital from Blackstone will advance Solrikitug in multiple indications.
"Our investment in Uniquity Bio illustrates Blackstone Life Sciences' commitment to finding, developing, and delivering potentially transformative medicines to patients around the world," said Nicholas Galakatos, Ph.D., Global Head of Blackstone Life Sciences, in a press release on May 15, 2024.
"We are proud to partner with Uniquity's team of veteran industry leaders as they advance solrikitug and expand their immunology and inflammation pipeline with additional programs in the near future."
The company aims to deliver best-in-class efficacy with solrikitug across several critical respiratory and GI indications with significant unmet needs. In the next month, Uniquity Bio will launch Phase 2 clinical trials in chronic obstructive pulmonary disease — the third leading cause of death worldwide, according to the World Health Organization — and asthma, which the WHO estimates affects more than 260 million people across the globe.