Search API
Following the initial shortage of the newly approved long-acting, monoclonal antibody Beyfortus™ (nirsevimab) for preventing infant respiratory syncytial virus (RSV) lower respiratory tract infection (LRTI), a new digital app may help identify newborns at the highest risk for developing serious RSV LRTI, according to research published at the ATS 2024 International Conference.
These researchers wrote, 'To predict whether these infants developed severe RSV LRTI requiring ICU admission during the first year of life, we developed a multivariable logistic regression model. The model includes demographic and clinical variables collected at or shortly after birth–19 variables, such as prenatal smoking, delivery method, maternal age, and assisted breathing (ventilation) during birth hospitalization.'
"Timely identification of infants at highest risk of RSV-related morbidity is key to prevention," said lead author Brittney M. Snyder, PhD, assistant professor, Division of Allergy, Pulmonary and Critical Care Medicine, Vanderbilt University Medical Center, in a press release on May 21, 2024.
"Our personalized risk prediction tool may have applications in allocating expensive and/or limited immunoprophylaxis (immunization with nirsevimab or palivizumab) to achieve the greatest benefit and promote RSV prevention among families with high-risk infants."
During the 2023-2024 RSV peak season in the U.S., Beyfortus reduced RSV hospitalizations by about 82% in infants compared to infants who received no passive immunization against RSV.
As of March 2024, among females with an infant <8 months, 41.3% reported that their infant received Beyfortus.
As of May 2024, access to Beyfortus is not constrained in the U.S.
The Montgomery County Department of Health and Human Services Office of Public Health recently reported one measles case in Philadelphia and potential virus exposures in Montgomery County, PA.
On May 20, 2024, the Philadelphia Department of Public Health (PDPH) reported one confirmed case of measles, which includes exposures at CVS Pharmacy, 10901C Bustleton Ave, Philadelphia, PA 19116, and Holy Redeemer Hospital Emergency Department, 1648 Huntingdon Pike, Strauss Emergency Pavilion, Meadowbrook, PA 19046.
Currently, there are no confirmed cases in Montgomery County.
"We believe there is no threat to the general public associated with this case of measles," said Pennsylvania Department of Health Acting Secretary Dr. Debra Bogen in a press release.
"Many countries, including travel destinations, are experiencing measles outbreaks, so the potential for travel-related measles cases and subsequent outbreaks in the United States has increased."
"We strongly encourage parents to follow the CDC's immunization schedule and get their children fully vaccinated as soon as they are able."
Previously, the PDPH declared the December 2023 - January 2024 measles outbreak to be over in February. Eight Philadelphians and one person outside Philadelphia tested positive for measles during this outbreak.
The outbreak did not spread further because 93% of Philadelphians are up-to-date on their MMR vaccine.
As of May 16, 2024, 21 U.S. jurisdictions had reported 139 measles cases. Throughout 2024, the City of Chicago confirmed the most measles cases (66) in the U.S.

Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) today announced it would award $3.96 million to the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine to develop a maternal vaccine that prevents sepsis caused by Klebsiella pneumoniae in newborns and infants.
The CVD vaccine candidate, which targets the surface sugars of K. pneumoniae, is expected to be given to pregnant women, thereby protecting their babies from this deadly infection.
If successful, researchers expect the vaccine will prevent 80-90% of K. pneumoniae neonatal sepsis infections.
“With this award, the CVD team has even greater potential to bring a maternal vaccine for neonatal sepsis to market and save the lives of millions of infants worldwide, especially in low-and-middle income countries through its global partnership with Auro Vaccines of India,” said Erin Duffy, PhD, R&D Chief of CARB-X, in a press release on May 21, 2024.
“By vaccinating expectant mothers, the risk of neonatal sepsis might be reduced substantially as babies who are too young to be vaccinated would receive antibodies from their mothers in utero as well as through breastfeeding after birth.”
The CARB-X award supports examining the feasibility of this project to maximize its potential impact on the vulnerable patient population.
The CVD team aims to develop and evaluate their vaccine further in partnership with Auro Vaccines Private Limited, a step-down subsidiary of Aurobindo Pharma Limited, India.
Neonatal sepsis is a life-threatening response to bloodstream infections that occur in newborns fewer than 28 days old. Due to their immature immune systems, newborns are particularly susceptible to infections.
The BARNARDS study estimated that 2.5 million neonates or infants in the first month of life die annually of sepsis, with the most significant burden in low- and middle-income countries.
Since neonatal sepsis progresses rapidly, it requires immediate treatment with IV fluids and antibiotics. The risk of death from neonatal sepsis increases by 7.6% every hour a treatment is delayed.
Federal funds from the U.S. Department of Health and Human Services, the Administration for Strategic Preparedness and Response, and others support CARB-X funding for this research.

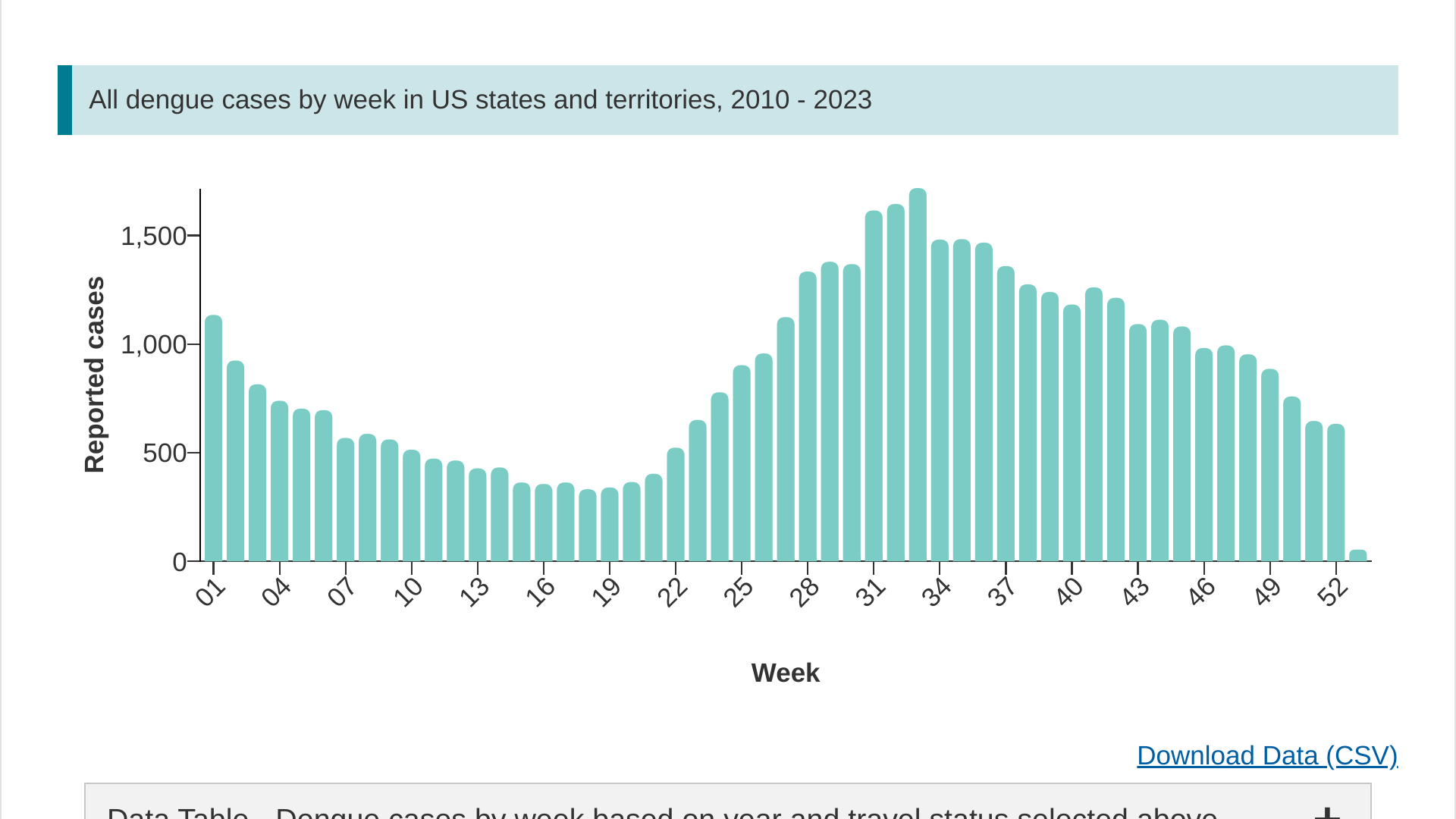
The Centers for Disease Control and Prevention (CDC) recently reported a significant increase in dengue disease cases in the U.S. Almost half of the world's population, about 4 billion people, live in areas at risk for dengue outbreaks in 2024.
On May 13, 2024, the CDC confirmed 1,495 dengue cases reported from 39 U.S. jurisdictions. Last year, 2,932 dengue cases were confirmed in the U.S.
Over the past 13 years, most dengue cases have been reported during week #33, which is in August.
While the Dengvaxia® vaccine is U.S. FDA-approved, the CDC says it is not approved for use in U.S. travelers who are visiting but not living in an area where dengue is common.
Globally, dengue vaccines are available under specific criteria.
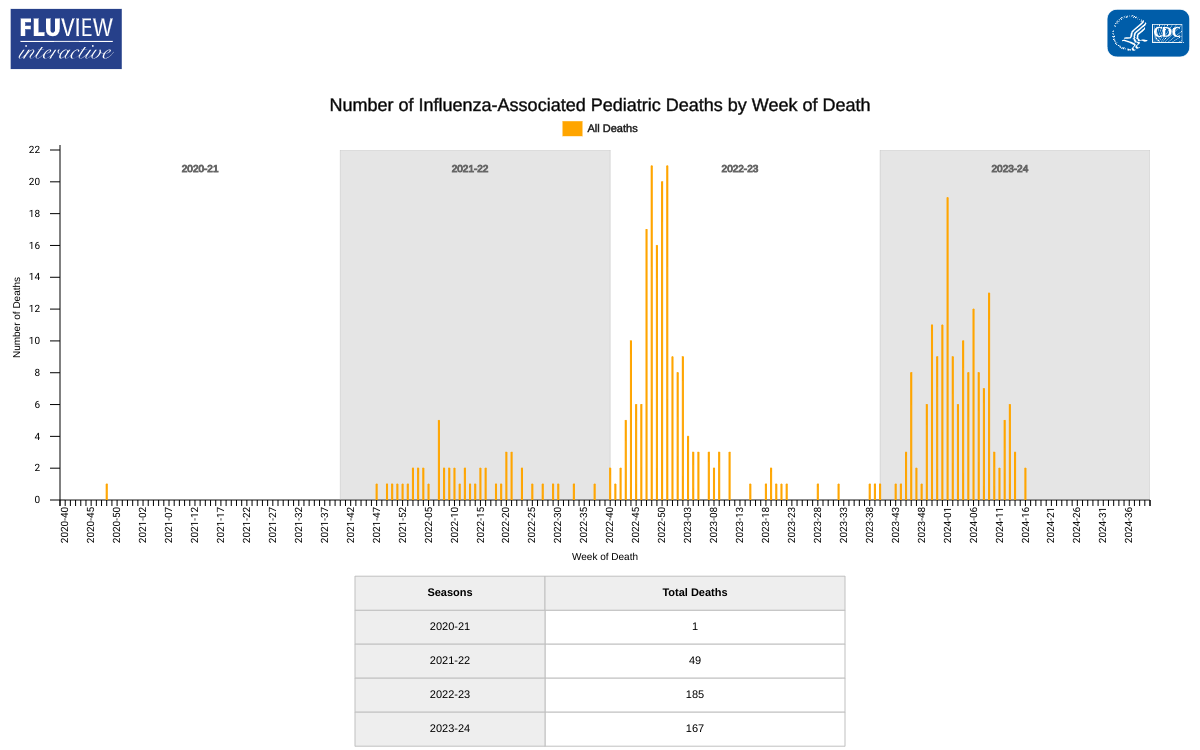
The U.S. Centers for Disease Control and Prevention (CDC) recently reported a decrease in pediatric deaths related to influenza infections.
As of May 17, 2024, the CDC reported three additional influenza-associated pediatric deaths during Week #19, bringing the total number of pediatric deaths for this season to 167.
Two deaths were associated with influenza A viruses, and one was associated with an influenza B/Victoria virus. One of the influenza A viruses had subtyping performed and was an A(H3N2) virus.
Last flu season, the CDC reported 187 pediatric deaths related to influenza.
The CDC recommends that everyone six months and older get an annual flu vaccine as long as flu activity continues in their area.
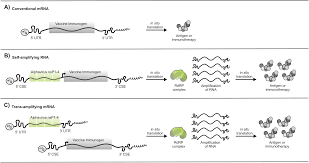
CSL and Arcturus Therapeutics today announce the journal Nature Communications has published results from an integrated phase 1/2/3a/3b study evaluating the safety, immunogenicity, and efficacy of ARCT-154, a novel self-amplifying (sa-mRNA) COVID-19 vaccine.
ARCT-154 is the world's first approved sa-mRNA COVID-19 vaccine for use in Japan.
The joint study's results demonstrate that two 5 μg doses of ARCT-154, sa-mRNA vaccine, were well-tolerated, immunogenic, and provided significant protection against multiple strains of COVID-19.
The efficacy of ARCT-154 against severe COVID-19 was 100% in healthy persons aged 18-59 and more than 90% in persons at risk of severe consequences of the disease due to co-morbidities or older age.
"The results published in Nature Communications demonstrate the efficacy and tolerability of ARCT-154 and add to a growing body of evidence that our sa-mRNA vaccine has the potential to provide significant protection against the pervasive virus, reinforcing our promise to protect public health," said Jon Edelman, M.D., Senior Vice President, Vaccines Innovation Unit, CSL, in a press release on May 20, 2024.
Unlike standard mRNA vaccines, sa-mRNA vaccines instruct the body to make more mRNA and protein to boost the immune response.
CSL is a global biotechnology company with a portfolio of medicines and vaccines.