Search API
The U.S. Centers for Disease Control and Prevention (CDC) recently reported a decrease in pediatric deaths related to influenza infections.
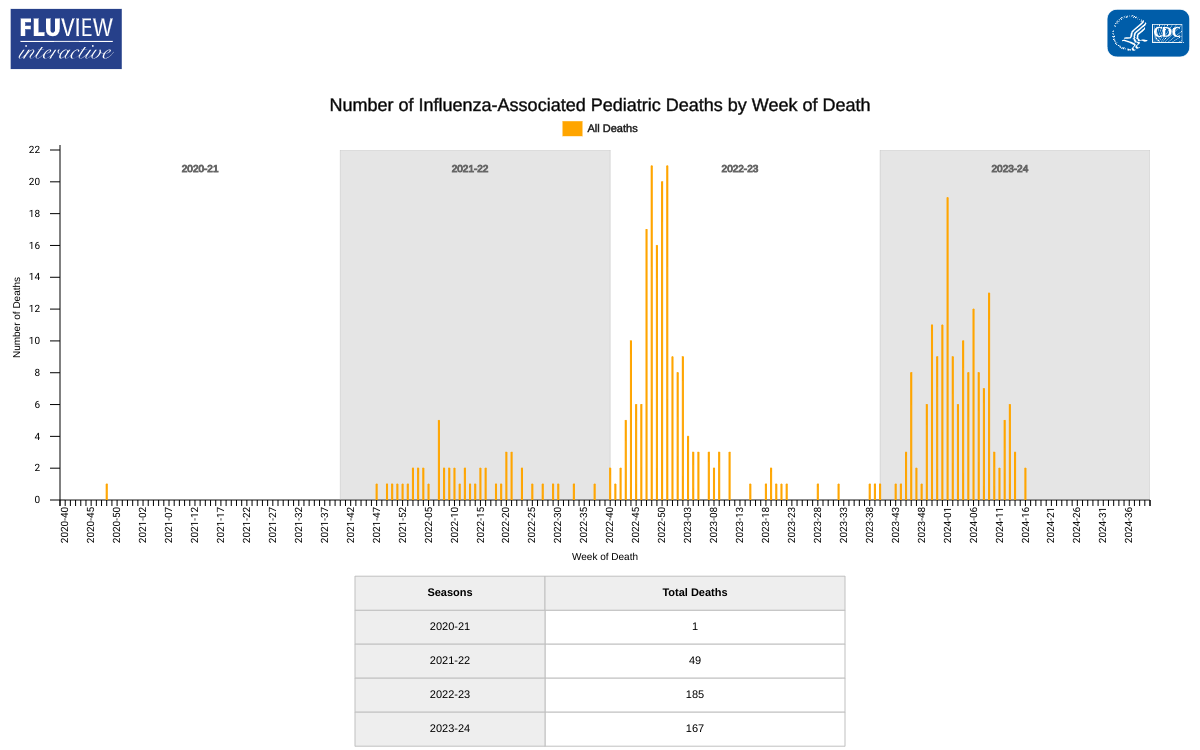
As of May 17, 2024, the CDC reported three additional influenza-associated pediatric deaths during Week #19, bringing the total number of pediatric deaths for this season to 167.
Two deaths were associated with influenza A viruses, and one was associated with an influenza B/Victoria virus. One of the influenza A viruses had subtyping performed and was an A(H3N2) virus.
Last flu season, the CDC reported 187 pediatric deaths related to influenza.
The CDC recommends that everyone six months and older get an annual flu vaccine as long as flu activity continues in their area.
CSL and Arcturus Therapeutics today announce the journal Nature Communications has published results from an integrated phase 1/2/3a/3b study evaluating the safety, immunogenicity, and efficacy of ARCT-154, a novel self-amplifying (sa-mRNA) COVID-19 vaccine.
ARCT-154 is the world's first approved sa-mRNA COVID-19 vaccine for use in Japan.
The joint study's results demonstrate that two 5 μg doses of ARCT-154, sa-mRNA vaccine, were well-tolerated, immunogenic, and provided significant protection against multiple strains of COVID-19.
The efficacy of ARCT-154 against severe COVID-19 was 100% in healthy persons aged 18-59 and more than 90% in persons at risk of severe consequences of the disease due to co-morbidities or older age.
"The results published in Nature Communications demonstrate the efficacy and tolerability of ARCT-154 and add to a growing body of evidence that our sa-mRNA vaccine has the potential to provide significant protection against the pervasive virus, reinforcing our promise to protect public health," said Jon Edelman, M.D., Senior Vice President, Vaccines Innovation Unit, CSL, in a press release on May 20, 2024.
Unlike standard mRNA vaccines, sa-mRNA vaccines instruct the body to make more mRNA and protein to boost the immune response.
CSL is a global biotechnology company with a portfolio of medicines and vaccines.
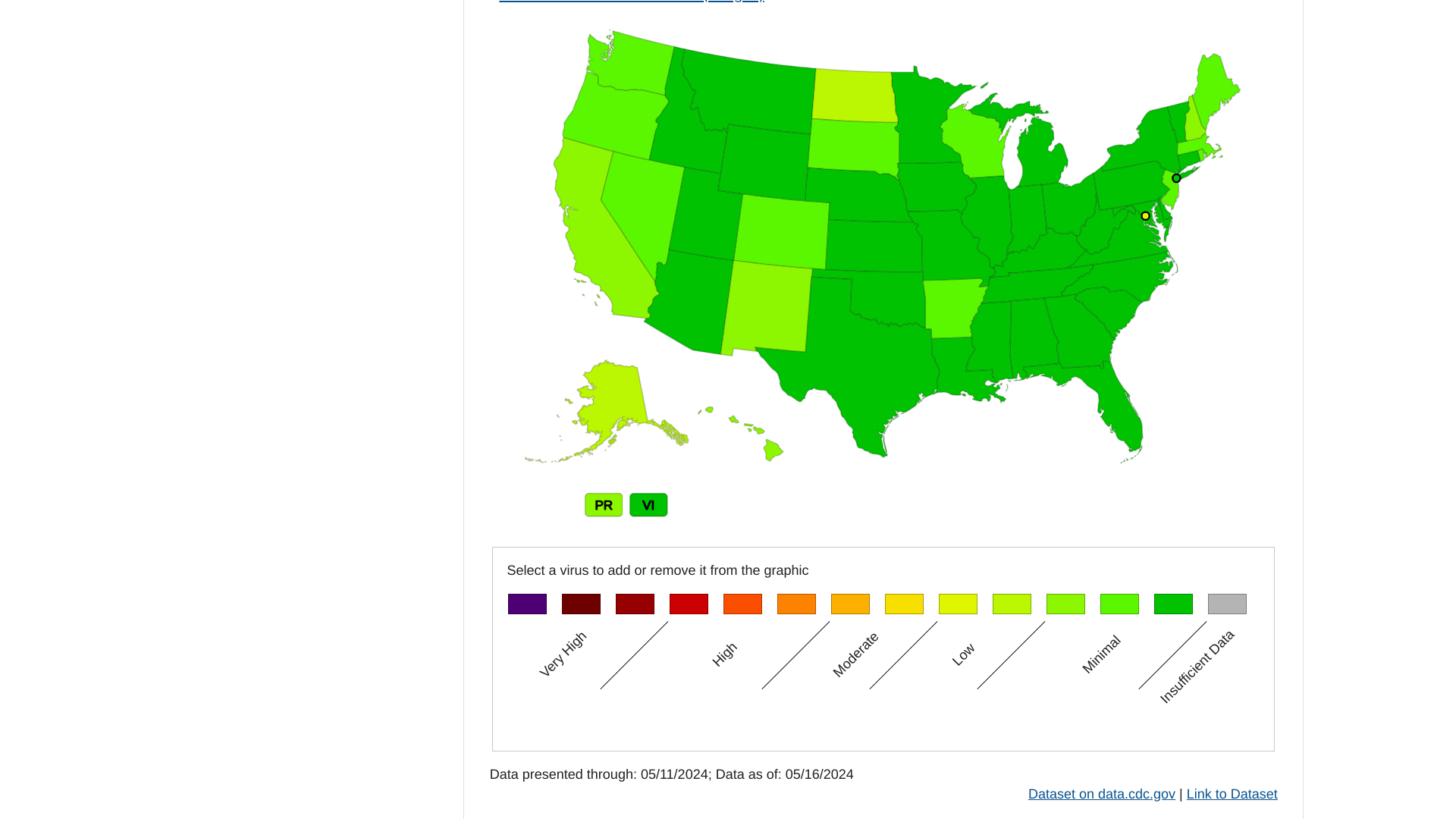
According to the U.S. Centers for Disease Control and Prevention (CDC), the amount of respiratory illness (fever plus cough or sore throat) causing people to seek healthcare is low nationally.
As of May 17, 2024, the CDC says no jurisdictions experienced moderate, high, or very high activity last week.
Furthermore, nationally, emergency department visits with diagnosed COVID-19, influenza, and RSV are at low levels.
While there are approved vaccines that help prevent these diseases, the CDC suggests speaking with a healthcare professional about the best time to be immunized.
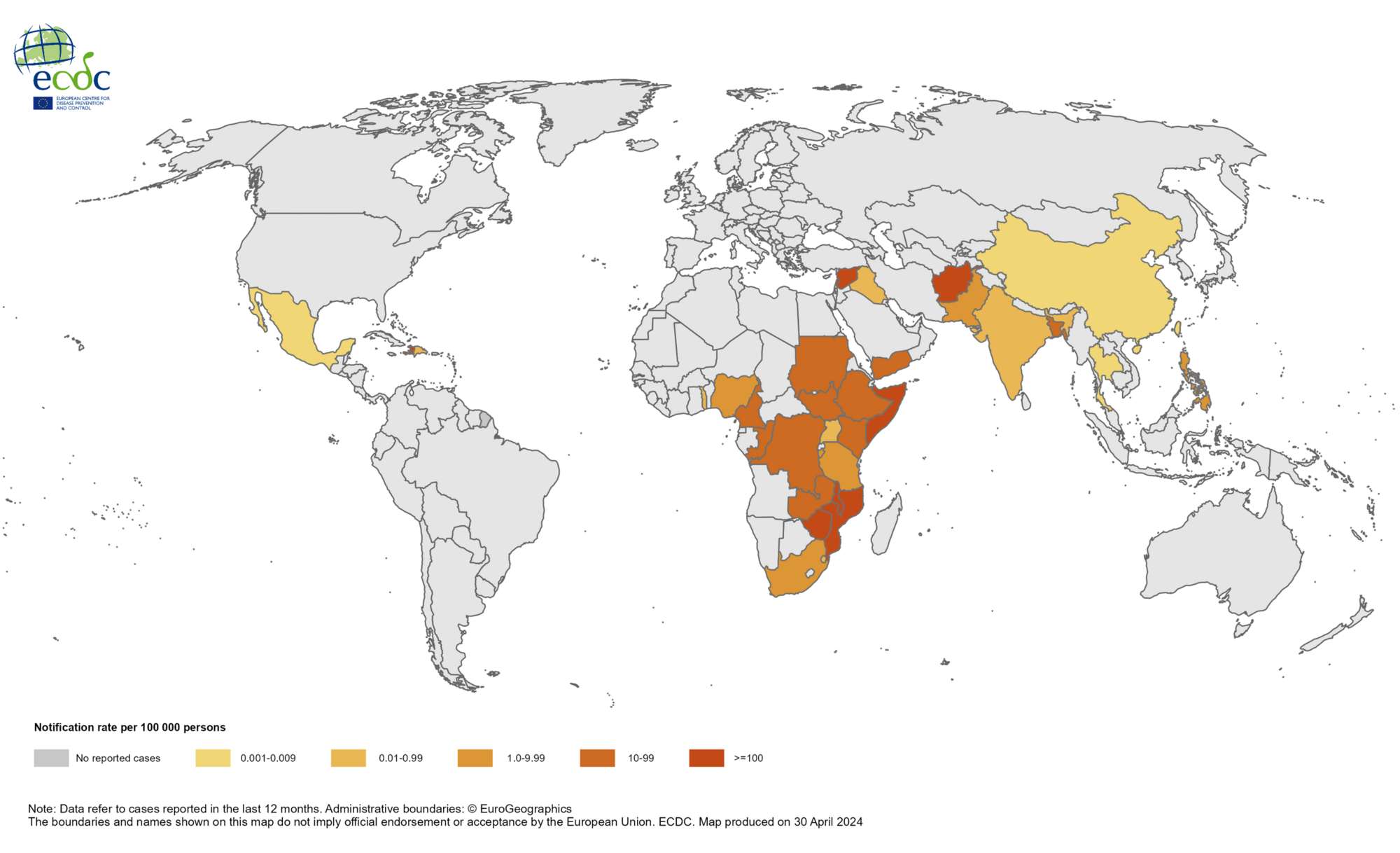
The World Health Organization (WHO) has reported a critical shortage of Oral Cholera Vaccines (OCV), which is affecting cholera outbreak responses.
As of May 16, 2024, the WHO's External Situation Report #14 classified the global resurgence of Cholera as a grade 3 emergency, its highest internal level for emergencies, due to the increasing number of outbreaks and their geographic spread, as well as the shortage of vaccines and other resources,
Since January 2023, OCV requests have surged, with 15 countries requesting 82 million doses, nearly double the 46 million doses produced during this period.
As of early May 2024, the OCV vaccine stockpile had 3.2 million doses, below the global target of five million.
From January 2024 to April 2024, a cumulative total of 145,900 cholera cases and 1,766 deaths were reported from 24 countries across five WHO regions.
No cholera outbreaks were reported in the United States.
The U.S. CDC says Cholera is an infection of the intestines caused by the bacterium Vibrio cholerae. You can get Cholera from drinking water or eating food containing cholera bacteria.
Most people who get Cholera don't get sick. However, Cholera can cause life-threatening watery diarrhea and vomiting.
As of May 2024, there are various OCVs available.
Two doses of OCV protect against Cholera for at least three years. Since October 2022, only one dose of vaccine has been used in outbreak response, offering protection for about a year.
In the U.S., cholera vaccines are generally available at travel clinics and certified pharmacies.
During the early stages of the recent pandemic, AstraZeneca's Evusheld antibody was an effective therapeutic option. However, as the SARS-CoV coronavirus evolved, the U.S. FDA withdrew Evusheld's authorization in early 2023.
According to the company's press release on May 16, 2024, positive high-level results from the SUPERNOVA Phase III COVID-19 pre-exposure prophylaxis (prevention) trial showed sipavibart (AZD3152), an investigational long-acting antibody (LAAB), demonstrated a statistically significant reduction in the incidence of symptomatic COVID‑19 compared to control in an immunocompromised patient population.
SUPERNOVA is a large Phase III global trial providing the only efficacy data in immunocompromised patients, demonstrating the potential benefit of a COVID-19 antibody against recent SARS-CoV-2 variants.
Sipavibart was well tolerated in the trial, and preliminary analyses showed that adverse events were balanced between the control and sipavibart arms.
Iskra Reic, Executive Vice President, Vaccines and Immune Therapies, AstraZeneca, commented, "Immunocompromised patients currently have limited or no options for COVID-19 protection and continue to face a significant burden of disease, despite often being fully vaccinated."
"Sipavibart has the potential to prevent COVID-19 in the immunocompromised, and we will now work with regulatory authorities globally to bring sipavibart to these vulnerable patients."
Sipavibart is not a preventive vaccine.
It was engineered using the same antibody scaffold as Evusheld and optimized with the same half-life extension, reduced Fc effector function, and complemented the C1q binding platform.
The reduced Fc effector function aims to minimize the risk of antibody-dependent disease enhancement - a phenomenon in which virus-specific antibodies promote, rather than inhibit, infection and/or disease.
According to the press release, AstraZeneca is in dialogue with regulatory authorities on potential authorization or approval pathways.

Eisai Co., Ltd. and Biogen Inc. recently announced the initiation of a rolling submission of a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for LEQEMBI® subcutaneous autoinjector for weekly maintenance dosing.
According to the company's press release on May 14, 2024, the injection process requires less time than the intravenous (IV) formulation.
If approved by the FDA, the LEQEMBI autoinjector could be used to administer LEQEMBI at home or medical facilities.
LEQEMBI (lecanemab-irmb) is a humanized immunoglobulin gamma 1 monoclonal antibody. It is indicated for the treatment of Alzheimer's disease (AD) in patients with Mild Cognitive Impairment or mild dementia stage of disease.
LEQEMBI is now approved in the U.S., Japan, and China, and applications have been submitted for review in the European Union, Australia, Brazil, Canada, Hong Kong, Great Britain, India, Israel, Russia, Saudi Arabia, South Korea, Taiwan, Singapore and Switzerland.
Previously, Eisai submitted a Supplemental BLA for monthly LEQEMBI IV maintenance dosing to the FDA in March 2024.
According to the Alzheimer's Association's Alzheimer's Disease Facts and Figures, an estimated 6.5 million Americans aged 65 and older live with dementia due to AD.
As of May 16, 2024, the FDA has not approved a vaccine that prevents AD.




