Search API
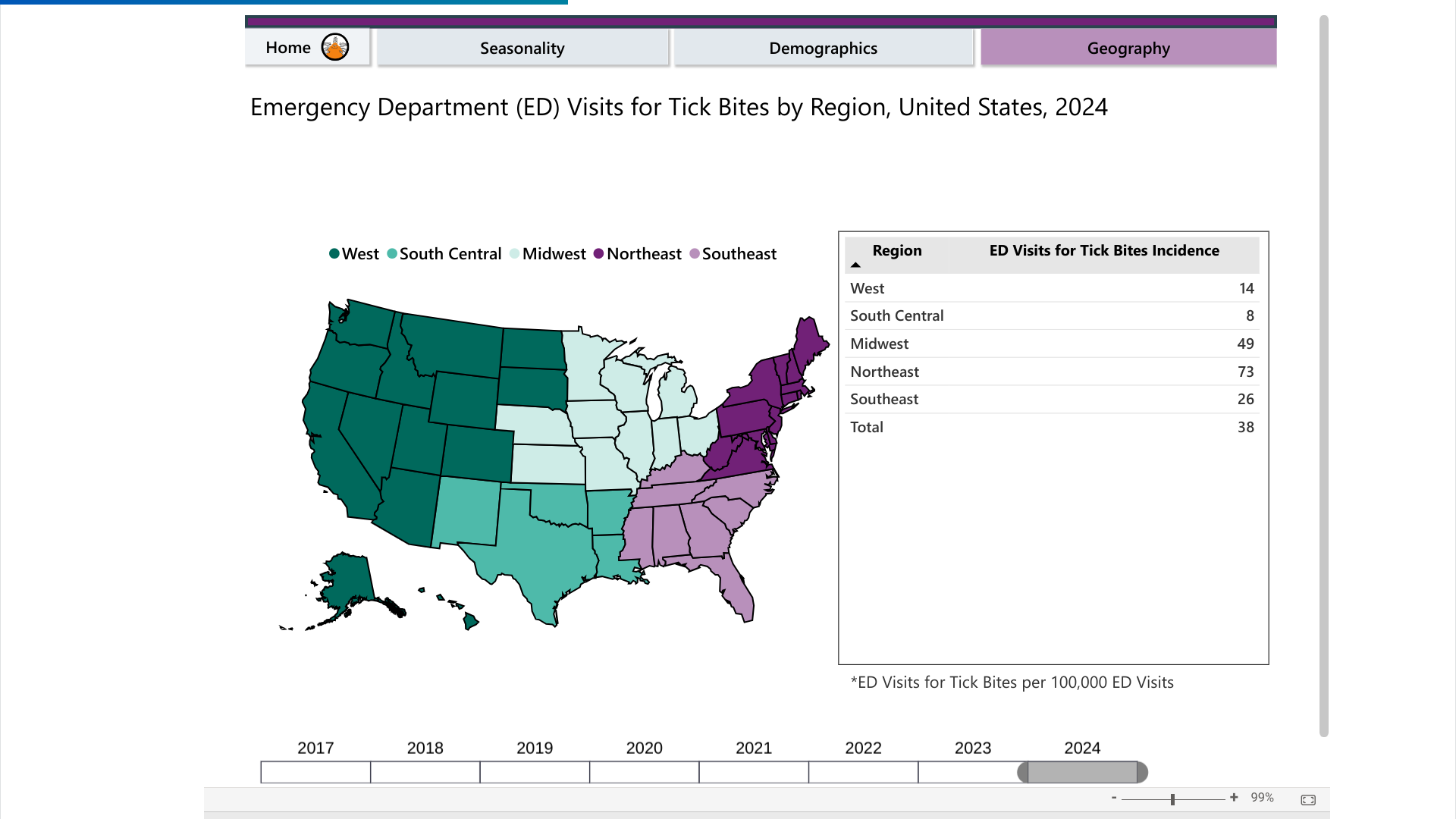
According to the U.S. CDC's Tick Bite Data Tracker dashboard, emergency room visits for tick bites have accelerated.
As of mid-May 2024, most tick disease cases in the U.S. have been concentrated in the Northeast, followed by the Midwest.
The CDC estimates that approximately 476,000 people are diagnosed and treated for Lyme disease yearly.
While Lyme disease received the most media attention, common Tickborne diseases in the United States include Babesiosis, Ehrlichiosis, and Rocky Mountain Spotted Fever.
While the Lyme disease vaccine candidate (VLA15) continues conducting late-stage research, it previously received the U.S. Food and Drug Administration Fast Track designation. The vaccine manufacturers have indicated updated study results in 2025.
Until a preventive Lyme disease vaccine becomes available, the CDC recommends protecting yourself from ticks by applying DEET or picaridin, treating clothing with products containing 0.5% permethrin, and conducting thorough tick checks after outdoor activities.
The U.S. Centers for Disease Control and Prevention (CDC) recently highlighted two gateway cities as the unfortunate leaders in this year's dengue outbreaks.
According to New York City Health, travel-related dengue cases have increased among residents in recent years. As of May 21, 2024, the CDC confirmed 114 cases.
And the Florida Department of Health recently confirmed 173 travel-associated dengue cases, mainly by visitors from Brazil and Cuba, and 7 cases of locally acquired dengue were reported from two counties (Miami-Dade, Pasco).
In 2023, Florida reported 609 travel-associated dengue cases, and 186 people locally contracted dengue from the bites of infected mosquitoes.
Throughout the Americas, 7,327,521 suspected cases of dengue (an increase of 243% from 2023) and over 1,700 deaths have been registered in 2024.
While dengue is a vaccine-preventable disease, vaccinations are not generally offered in the U.S.

Assembly Biosciences, Inc. today announced that the company will present data from its herpes simplex virus (HSV) program at the International Herpesvirus Workshop in Portland, Ore., July 13-17, 2024.
At the Workshop, Assembly Bio will present data describing the preclinical profile of ABI-5366, a long-acting helicase-primase inhibitor candidate in development for treating recurrent genital herpes.
Additionally, the company will present data highlighting the preclinical characterization of ABI-1179, a structurally distinct long-acting helicase-primase inhibitor candidate.
William Delaney, PhD, chief scientific officer of Assembly Bio, commented in a press release on May 22, 2024, "Improved therapeutic options are urgently needed for people living with recurrent genital herpes, as the current standard of care is only partially effective in controlling recurrences."
"Our HSV program employs a highly innovative approach, with candidates designed from the start for long-acting administration and targeting the viral helicase-primase complex, a different viral target than the standard of care."
"We look forward to an important year for the program, as we remain on track to begin dosing in a Phase 1a/1b study for ABI-5366 by mid-year (2024) and anticipate bringing ABI-1179 into the clinic by the end of the year."
Details of the presentations are as follows:
Poster Presentation: The Helicase-Primase Inhibitor ABI-5366 is a Novel, Potent, Long-Acting Inhibitor for the Treatment of Recurrent Genital Herpes
Presenter: Ran Yan, PhD, Assembly Bio
Poster Session Date and Time: Not Yet Available
As of May 23, 2024, there are no U.S. FDA-approved herpes vaccines available.
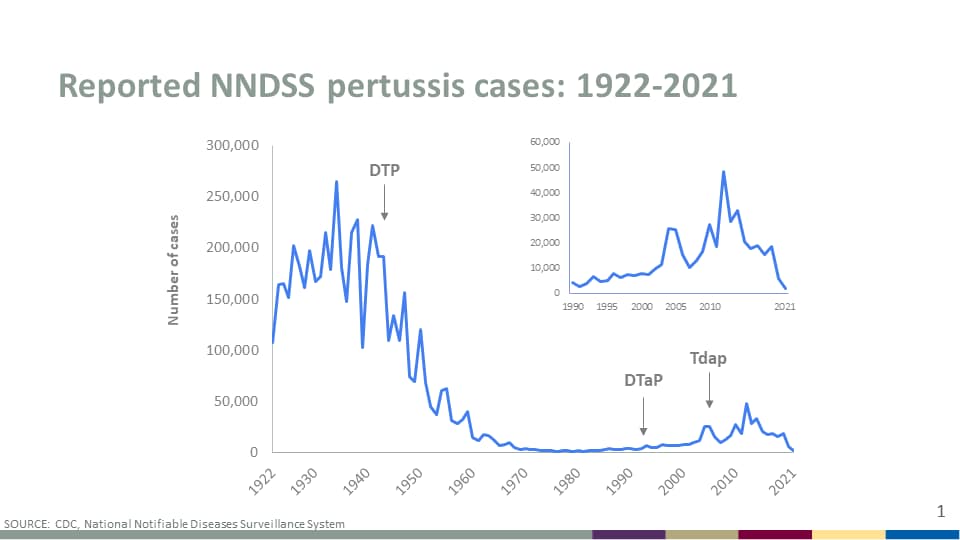
The Lexington-Fayette County Health Department recently announced a pertussis outbreak after 9 cases were confirmed since late April 2024. These cases include students attending local high schools.
“Any school-age children with symptoms of pertussis should stay home from school and visit their health care provider for evaluation, even if they have previously been vaccinated,” the health department said in a statement on May 20, 2024.
Pertussis (Whooping cough) is a contagious respiratory illness that spreads quickly from person to person. The health department recommends preventive antibiotics for high-risk students exposed to pertussis.
Kentucky requires school-age children to be vaccinated against pertussis. For protection, one dose of the Tdap vaccine is recommended for children ages 11 and above.
Throughout the United States, pertussis cases have been increasing in recent years.
The U.S. CDC reported 5,611 cases in 2023, significantly more than the 2,388 pertussis cases reported in 2022.
Internationally, pertussis outbreaks have continued in 2024.
According to the European Centre for Disease Prevention and Control (ECDC), more than 32,000 pertussis cases were reported between January and March 2024. The ECDC data announced on May 8, 2024, indicates a nearly 10-fold increase in pertussis cases this year.
Following the initial shortage of the newly approved long-acting, monoclonal antibody Beyfortus™ (nirsevimab) for preventing infant respiratory syncytial virus (RSV) lower respiratory tract infection (LRTI), a new digital app may help identify newborns at the highest risk for developing serious RSV LRTI, according to research published at the ATS 2024 International Conference.
These researchers wrote, 'To predict whether these infants developed severe RSV LRTI requiring ICU admission during the first year of life, we developed a multivariable logistic regression model. The model includes demographic and clinical variables collected at or shortly after birth–19 variables, such as prenatal smoking, delivery method, maternal age, and assisted breathing (ventilation) during birth hospitalization.'
"Timely identification of infants at highest risk of RSV-related morbidity is key to prevention," said lead author Brittney M. Snyder, PhD, assistant professor, Division of Allergy, Pulmonary and Critical Care Medicine, Vanderbilt University Medical Center, in a press release on May 21, 2024.
"Our personalized risk prediction tool may have applications in allocating expensive and/or limited immunoprophylaxis (immunization with nirsevimab or palivizumab) to achieve the greatest benefit and promote RSV prevention among families with high-risk infants."
During the 2023-2024 RSV peak season in the U.S., Beyfortus reduced RSV hospitalizations by about 82% in infants compared to infants who received no passive immunization against RSV.
As of March 2024, among females with an infant <8 months, 41.3% reported that their infant received Beyfortus.
As of May 2024, access to Beyfortus is not constrained in the U.S.
The Montgomery County Department of Health and Human Services Office of Public Health recently reported one measles case in Philadelphia and potential virus exposures in Montgomery County, PA.
On May 20, 2024, the Philadelphia Department of Public Health (PDPH) reported one confirmed case of measles, which includes exposures at CVS Pharmacy, 10901C Bustleton Ave, Philadelphia, PA 19116, and Holy Redeemer Hospital Emergency Department, 1648 Huntingdon Pike, Strauss Emergency Pavilion, Meadowbrook, PA 19046.
Currently, there are no confirmed cases in Montgomery County.
"We believe there is no threat to the general public associated with this case of measles," said Pennsylvania Department of Health Acting Secretary Dr. Debra Bogen in a press release.
"Many countries, including travel destinations, are experiencing measles outbreaks, so the potential for travel-related measles cases and subsequent outbreaks in the United States has increased."
"We strongly encourage parents to follow the CDC's immunization schedule and get their children fully vaccinated as soon as they are able."
Previously, the PDPH declared the December 2023 - January 2024 measles outbreak to be over in February. Eight Philadelphians and one person outside Philadelphia tested positive for measles during this outbreak.
The outbreak did not spread further because 93% of Philadelphians are up-to-date on their MMR vaccine.
As of May 16, 2024, 21 U.S. jurisdictions had reported 139 measles cases. Throughout 2024, the City of Chicago confirmed the most measles cases (66) in the U.S.

Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) today announced it would award $3.96 million to the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine to develop a maternal vaccine that prevents sepsis caused by Klebsiella pneumoniae in newborns and infants.
The CVD vaccine candidate, which targets the surface sugars of K. pneumoniae, is expected to be given to pregnant women, thereby protecting their babies from this deadly infection.
If successful, researchers expect the vaccine will prevent 80-90% of K. pneumoniae neonatal sepsis infections.
“With this award, the CVD team has even greater potential to bring a maternal vaccine for neonatal sepsis to market and save the lives of millions of infants worldwide, especially in low-and-middle income countries through its global partnership with Auro Vaccines of India,” said Erin Duffy, PhD, R&D Chief of CARB-X, in a press release on May 21, 2024.
“By vaccinating expectant mothers, the risk of neonatal sepsis might be reduced substantially as babies who are too young to be vaccinated would receive antibodies from their mothers in utero as well as through breastfeeding after birth.”
The CARB-X award supports examining the feasibility of this project to maximize its potential impact on the vulnerable patient population.
The CVD team aims to develop and evaluate their vaccine further in partnership with Auro Vaccines Private Limited, a step-down subsidiary of Aurobindo Pharma Limited, India.
Neonatal sepsis is a life-threatening response to bloodstream infections that occur in newborns fewer than 28 days old. Due to their immature immune systems, newborns are particularly susceptible to infections.
The BARNARDS study estimated that 2.5 million neonates or infants in the first month of life die annually of sepsis, with the most significant burden in low- and middle-income countries.
Since neonatal sepsis progresses rapidly, it requires immediate treatment with IV fluids and antibiotics. The risk of death from neonatal sepsis increases by 7.6% every hour a treatment is delayed.
Federal funds from the U.S. Department of Health and Human Services, the Administration for Strategic Preparedness and Response, and others support CARB-X funding for this research.

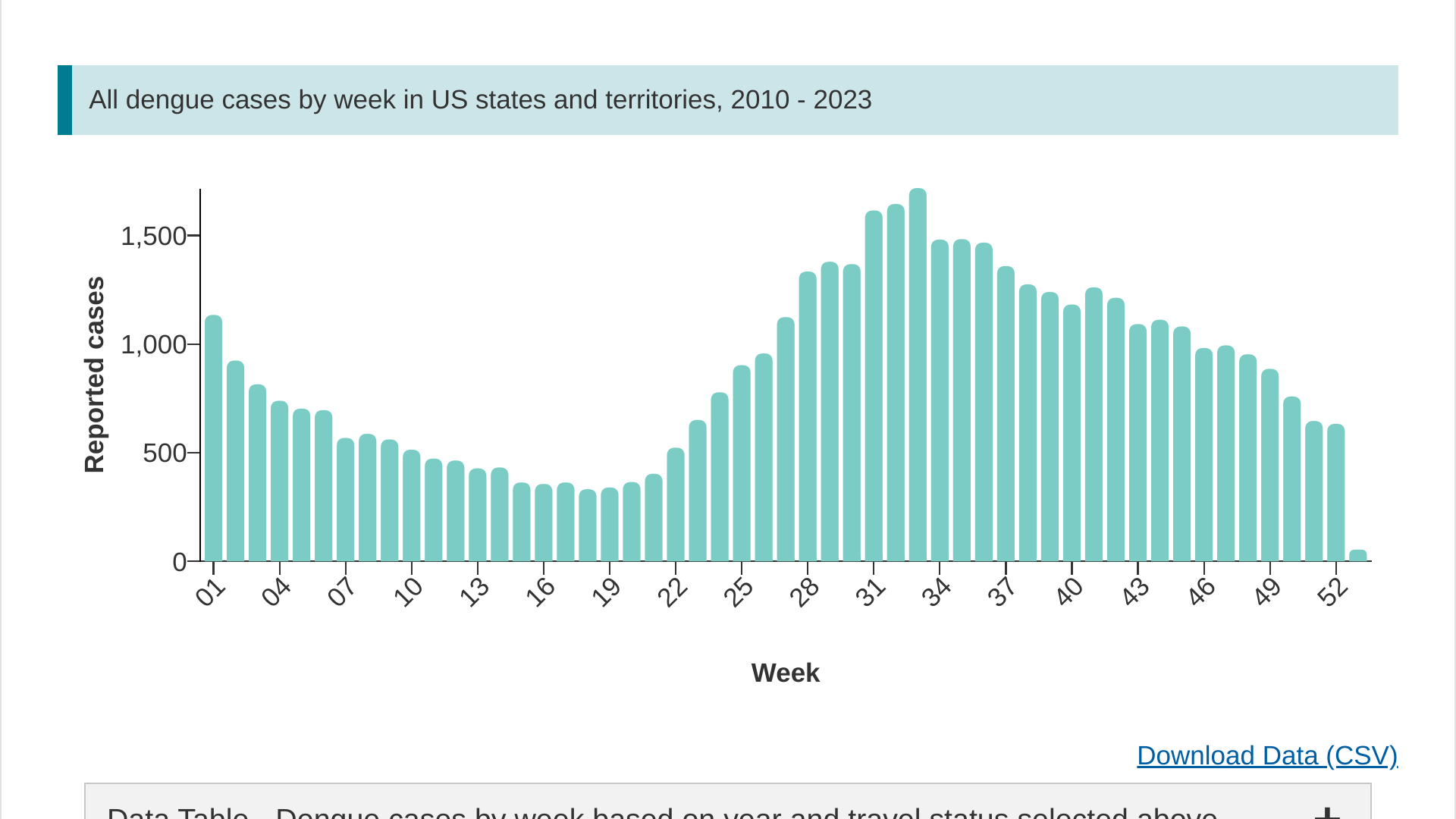
The Centers for Disease Control and Prevention (CDC) recently reported a significant increase in dengue disease cases in the U.S. Almost half of the world's population, about 4 billion people, live in areas at risk for dengue outbreaks in 2024.
On May 13, 2024, the CDC confirmed 1,495 dengue cases reported from 39 U.S. jurisdictions. Last year, 2,932 dengue cases were confirmed in the U.S.
Over the past 13 years, most dengue cases have been reported during week #33, which is in August.
While the Dengvaxia® vaccine is U.S. FDA-approved, the CDC says it is not approved for use in U.S. travelers who are visiting but not living in an area where dengue is common.
Globally, dengue vaccines are available under specific criteria.