Search API
With one approved chikungunya vaccine already available, the U.S. Food and Drug Administration (FDA) has accepted and granted Priority Review for the Biologics License Application (BLA) for Bavarian Nordic A/S CHIKV VLP, a vaccine candidate for immunization to prevent disease outbreaks caused by chikungunya virus infection in individuals 12 years of age and older.
The Priority Review designation means the FDA aims to complete its review within six months. The FDA has assigned a target action date for the Prescription Drug User Free Act of February 14, 2025.
CHIKV VLP is an adjuvanted VLP-based vaccine candidate for active immunization to prevent disease caused by CHIKV infection.
Paul Chaplin, President and CEO of Bavarian Nordic, said in a press release on August 13, 2024, “The FDA review, along with the ongoing review of our CHIKV VLP vaccine by the European Medicines Agency, represent the first regulatory reviews of a chikungunya vaccine for adolescents, potentially providing a broader usage by populations at risk of this debilitating disease.”
CHIKV VLP is currently also under accelerated assessment review with the EMA, potentially supporting approval of the vaccine by the European Commission in the first half of 2025.
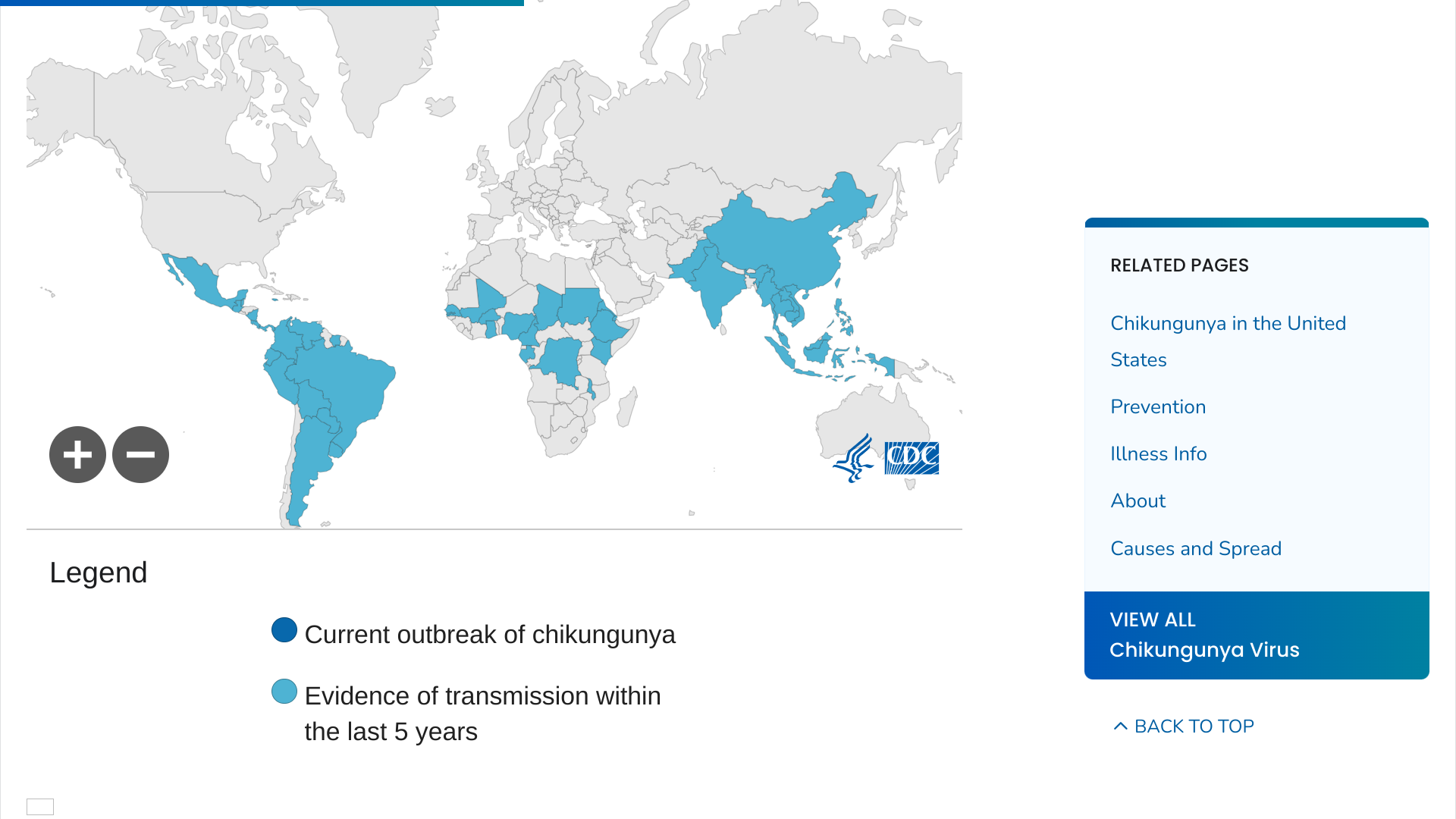
Chikungunya is a mosquito-borne viral disease caused by the chikungunya virus (CHIKV). The disease typically presents with acute symptoms, including fever, rash, fatigue, headache, and often severe and incapacitating joint pain.
While mortality is relatively low, morbidity is high; nearly 50% of individuals with CHIKV disease have debilitating long-term symptoms that can intensify with age.
Over the past few decades, CHIKV has emerged in several previously non-endemic regions in Asia, Africa, southern Europe, and the Region of the Americas, often causing large, unpredictable outbreaks.
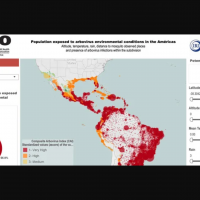
As of August 8, 2024, the Pan American Health Organization (PAHO) reported over 371,167 CHIKV cases in the Americas this year. Between 2013 and 2023, the PAHO reported more than 3.7 million CHIKV cases in the Americas.
The U.S. CDC reported from 2006 to 2023, 4,590 travel-related CHIKV cases were reported in the U.S., in areas such as Florida.
However, Locally acquired cases have not been reported in U.S. states or territories since 2019.
Pfizer Inc. today announced positive top-line safety and immunogenicity results from substudy B of the ongoing pivotal Phase 3 clinical trial.
The trial is evaluating two doses of the ABRYSVO™ vaccine in immunocompromised adults aged 18 and older at risk of developing severe respiratory syncytial virus (RSV)- associated lower respiratory tract disease.
ABRYSVO was well-tolerated during the trial, showing a safety profile consistent with findings from other vaccine studies.
While the company evaluated two doses, a single 120 µg dose of ABRYSVO generated a strong neutralizing response against both subtypes of RSV, RSV-A, and RSV-B across all cohorts and age groups in the study.
Pfizer plans to share these findings at an upcoming scientific conference, publish them in a peer-reviewed scientific journal, and submit the data to the regulatory agencies for review.
“Immunocompromised adults, such as patients with cancer or autoimmune disorders, have a substantially increased risk of experiencing severe complications from RSV, yet there are currently no vaccines approved for those aged 18 to 59 in the U.S.,” said Annaliesa Anderson, Ph.D., Senior Vice President and Chief Scientific Officer, Vaccine Research and Development, Pfizer, in a press release on August 12, 2024.
“We are encouraged by the positive top-line data from this study, which provide important evidence that ABRYSVO has the potential to address a significant unmet need in this vulnerable population.”
These most recent data in immunocompromised adults build on the body of evidence supporting the profile of ABRYSVO in high-risk adults.
As of early August 2024, three RSV vaccines have been approved for use in the U.S., and several vaccine candidates are conducting late-stage studies.
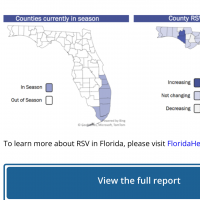
Additionally, the U.S. CDC reported on August 9, 2024, that RSV sections were generally low, except for the state of Florida.
At the end of July 2024, Swissmedic authorized Takeda Pharma AG's QDENGA® (TAK-003) Tetravalent Vaccine (Live, Attenuated) vaccine after assessing its efficacy, safety, and quality.
According to Swissmedic's statement on August 2, 2024, QDENGA is authorized for people aged four years and older who travel to regions where dengue fever is prevalent. These areas primarily include subtropical and tropical regions in Central Africa, Latin America, India, and Southeast Asia.
Swissmedic says QDENGA cannot cause the disease, but vaccination triggers the immune system to defend the body against the virus. When a person receives the vaccine, their immune system recognizes the attenuated variants as foreign and forms antibodies against them. When they come into contact with the virus again, the body rapidly produces more antibodies to neutralize it before the person contracts dengue fever.
The U.S. Centers for Disease Control and Prevention (CDC) recently announced that the global incidence of dengue this year is the highest in recent history, with about 100 countries reporting higher-than-usual dengue cases.
In 2024, both travel-related and locally transmitted dengue infections were confirmed in the south of the USA, in states such as Florida (335) and Texas (18).
Dengue fever is a viral disease spread by infected mosquitoes. It usually develops between four and seven days after the person is bitten. Symptoms include fever, headache, pain behind the eyes, muscle and joint pain, nausea or vomiting, swollen glands, and rash.
As of August 21, 2024, QDENGA had been approved in 40 countries and launched (or was available) in 24 of them.
Note: This new article was updated with information on current vaccine availability with a changed headline on August 23, 204.
Throughout 2024, the Florida Department of Health has reported various mosquito-transmitted diseases infecting people. Florida has confirmed that 14 species of Anopheles mosquitoes transmit diseases to humans.
While Dengue fever gets most of the media headlines, diseases such as Chikungunya, Malaria, and West Nile virus have also been detected this year.
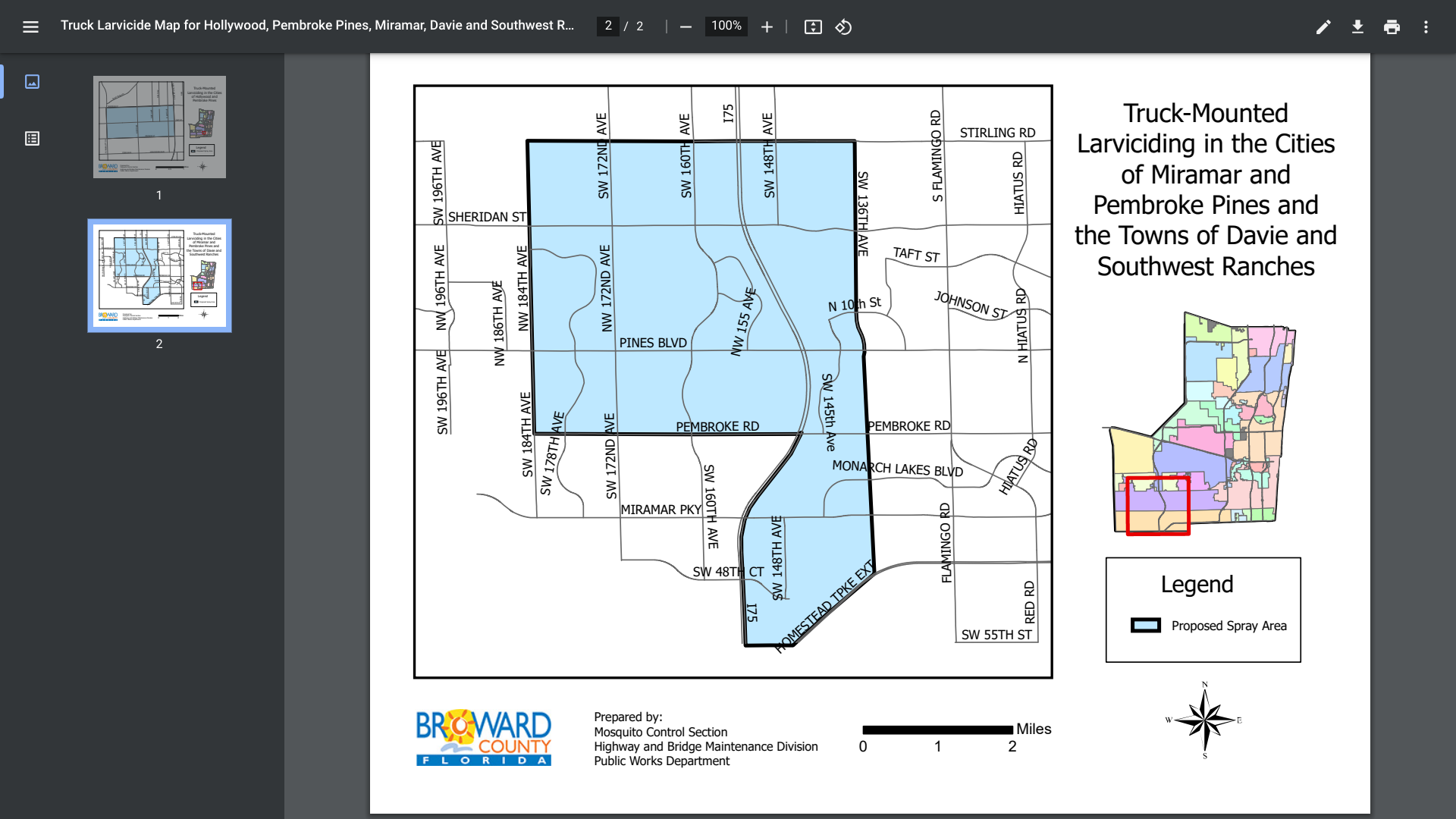
For example, on August 5, 2024, a locally acquired case of West Nile Virus was confirmed in Broward County.
As a direct response, the Broward County Mosquito Control Section recently announced it would spray organic larvicide as part of an integrated pest management approach. Truck-mounted larviciding will be performed between August 5 - 22, 2020.
This month, portions of Hollywood, Pembroke Pines, Davie, and Southwest Ranches are being targeted in southeast Florida.
The larvicide used is VectoBac WDG, which is not harmful to humans, pets, bees, aquatic habitats, or environmentally sensitive areas. The active ingredient is Bacillus thuringiensis israelensis (Bti, strain AM6552), a naturally occurring, biodegradable bacterial mosquito larvicide.
Broward County Mosquito Control Section continues to work closely with the Florida Department of Health and Code Enforcement partners in Broward's 31 municipalities to reduce the population of mosquitoes and their habitats. Any Broward County resident experiencing mosquito problems can request service by calling 311.
Regarding disease prevention, the U.S. Centers for Disease Control and Prevention (CDC) recently recommended Valneva SE's IXCHIQ®, a monovalent, single-dose, live-attenuated chikungunya vaccine. Vaccination is advised for international travelers since five chikungunya cases with onset in 2024 have been reported in individuals with a travel history to Brazil.
As of August 10, 2024, the CDC has not issued vaccination requirements for Florida residents or visitors.
According to the World Health Organization (WHO), the global Dengue fever outbreak continues to expand in August 2024.
Dengue outbreaks are being reported by 90 countries in 2024, with most of these cases reported in the Region of the Americas.
As of August 8, 2024, 43 countries and territories in the Region have reported over 11.1 million Dengue cases and about 6,135 related deaths this year.
The updated data is over 120% greater than recorded throughout 2023.
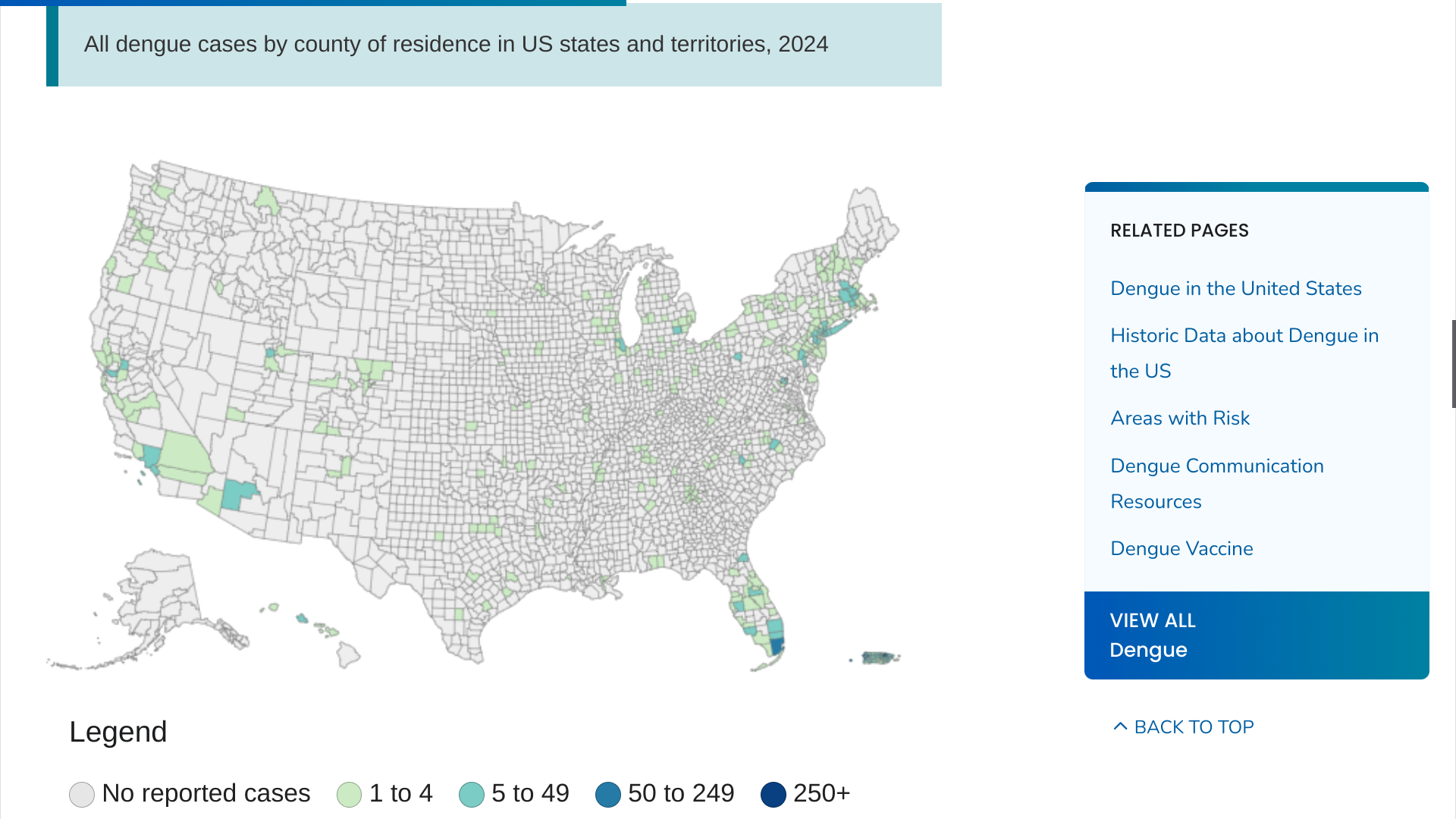
In the United States, the U.S. CDC reported on August 7, 2024, that 52 jurisdictions, led by Florida, New York/New Jersey, and Puerto Rico, had confirmed 3,290 dengue cases.
In 2023, only 2,343 Dengue cases were reported to the CDC.
The CDC says Dengue is endemic in the U.S. territories of Puerto Rico, American Samoa, the U.S. Virgin Islands, the Federated States of Micronesia, the Republic of Marshall Islands, and the Republic of Palau.
Currently, the CDC says the best way to prevent this mosquito-transmitted disease is to wear protective clothing, as no Dengue vaccine is available in the U.S.
However, in 2024, Takeda's QDENGA® (TAK-003) two-dose vaccine is available in over 20 countries. The WHO added QDENGA to its List of Prequalified Vaccines effective May 9, 2024.
The International Journal of Infectious Diseases recently published a new study conducted by Columbia University that revealed a 140% surge in measles cases worldwide from 2010 to 2019 across 194 member countries of the World Health Organization.
In a Short Communication dated July 22, 2024, the researchers highlighted that decreasing vaccination rates in 59 of the 194 nations were attributed to socio-economic issues in some less developed countries and vaccine hesitancy in wealthier nations.
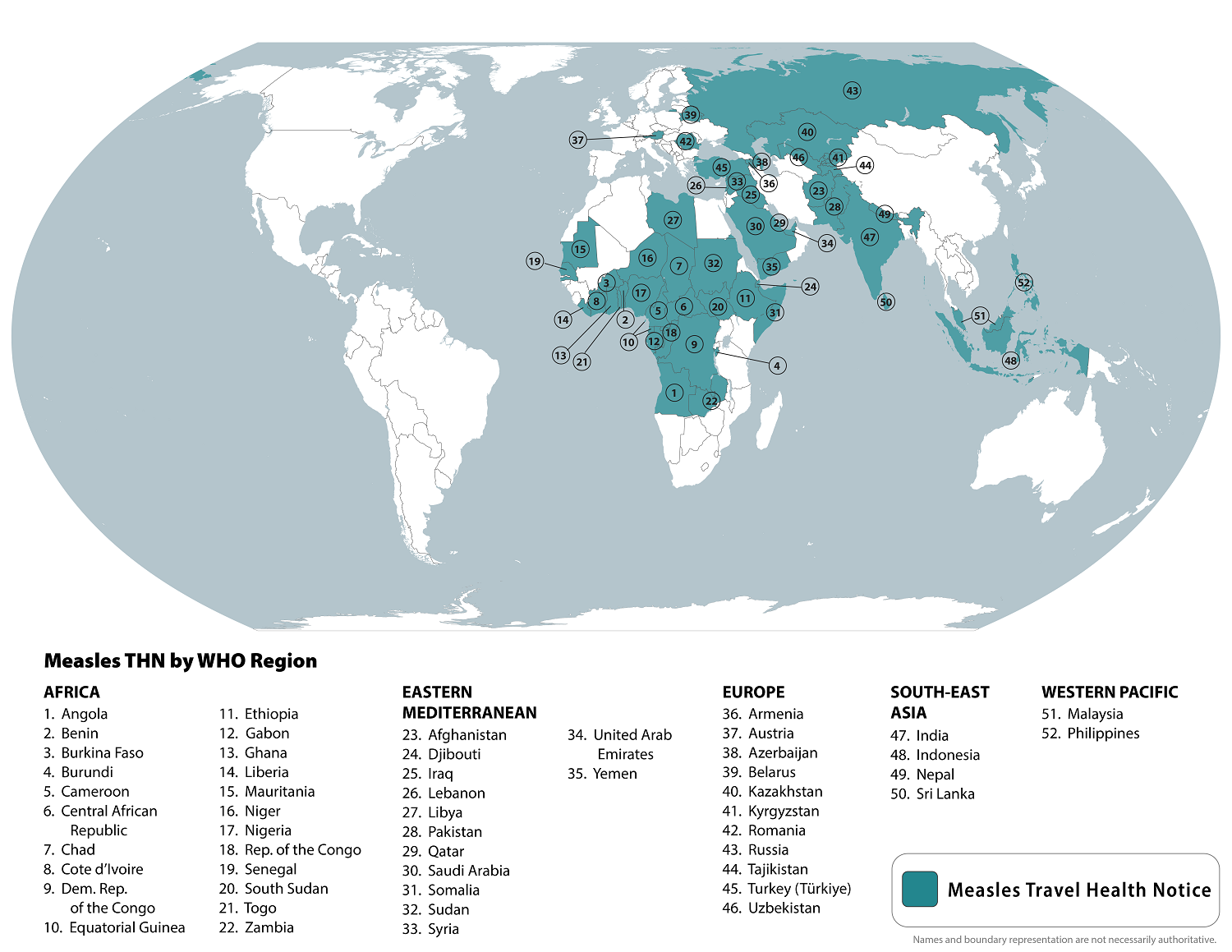
In July 2024, the U.S. Centers for Disease Control and Prevention (CDC) listed the top ten international measles outbreaks led by Azerbaijan, Kazakhstan, Iraq, and India. In total, the CDC listed 52 countries.
"Our analysis also suggests vaccine hesitancy may substantially contribute to the recent increases in measles incidence in wealthier countries. For instance, recent measles outbreaks in Europe and the United States have been linked to international travel and communities with prevailing vaccine skepticism,' wrote these researchers.
As of July 11, 2024, a total of 167 measles cases were reported by Arizona, California, Florida, Georgia, Illinois, Indiana, Louisiana, Maryland, Michigan, Minnesota, Missouri, New Hampshire, New Jersey, New Mexico, New York City, New York State, Ohio, Oregon, Pennsylvania, Vermont, Virginia, Washington, West Virginia, and Wisconsin.
The CDC reports 13 measles outbreaks in 2024, compared to 4 outbreaks reported in 2023.
Various U.S. FDA-approved measles vaccines are generally available at pharmacies in the United States.