Search API
According to data published by the U.S. Centers for Disease Control and Prevention (CDC), a UCLA-led multi-site study that included 45% women and 20% children, no mpox cases were reported,
During June–December 2023, among 196 patients in the study, only three mpox cases were identified (1.5%). All cases were among men who reported having sex with multiple men in the month prior and not being vaccinated against mpox.
The CDC's MMWR revealed on June 6, 2024, that clinicians should remain vigilant for mpox virus infections and educate patients about the importance of risk reduction and JYNNEOS® vaccination.
Bavarian Nordic produces the third generation JYNNEOS® (MVA-BN®, IMVAMUNE®), a two-dose vaccine based on a live, attenuated vaccinia virus, Modified Vaccinia Ankara.
A meta-analysis of 16 studies published on April 26, 2024, revealed that the JYNNEOS vaccine effectiveness (VE) for one pre-exposure prophylactic vaccination ranged from 35% to 86%, and VE ranged from 66% to 90% for two doses.
In 2024, JYNNEOS became commercially available in the U.S. Currently, the CDC does not recommend routine immunization against mpox for the general public and has not endorsed JYNNEOS booster doses (3rd).

Liberia is one African nation leading a crucial fight against polio. Unfortunately, Liberia recently reported a resurgence of variant polio type 2 in wastewater.
Liberia reported two variant polio type 2 detections from environmental sampling in 2023 and six so far in 2024.
Although historic polio vaccine coverage rates have shown promising progress, the battle against polio requires unwavering commitment and action, wrote the Global Polio Eradication Initiative on June 4, 2024.
Despite the challenges, the number of variant poliovirus cases in Liberia has significantly declined, with no children paralyzed by polio since 2021.
To stop the spread of the virus quickly, Liberia has launched nationwide immunization campaigns using the novel oral polio vaccine (nOPV2).
The second round of nOPV2 vaccinations is set to begin on June 7, 2024.
Primarily deployed in Africa during the last two years, the nOPV2 vaccine has been offered over 1 billion times.
Mr. Adolphus Clark, Expanded Programme on Immunization Manager in Liberia, expressed his optimism in a media release about the pending vaccination campaign, stating, “Our collective efforts have brought us closer than ever to a polio-free Liberia.... we are renewing our commitment to ensure that every child is protected from this preventable disease.”
Liberia has also introduced seven vaccines into its routine immunization schedule: Pneumococcal Conjugate Vaccine, Rotavirus, Inactive Polio Virus, Haemophilus influenzae Tue B, Typhoid, and measles-containing vaccine.
To alert international visitors, the U.S. CDC has included Liberia in its recent polio and measles Travel Health Advisories. The CDC suggests travelers speak with a travel vaccine expert about immunization options one month before visiting Liberia.

Auro Vaccines LLC's Nipah Virus vaccine candidate HeV attachment G glycoprotein (HeV-sG-V) was recently found to induce antibodies within one month of vaccination, and the persistence afforded by two dosages suggests the vaccine candidate has the potential for reactive Nipal outbreak control and preventative use.
On May 30, 2024, results from a Phase 1 study funded by the Coalition for Epidemic Preparedness Innovations and published by The Lancet preprint evaluated a recombinant subunit vaccine consisting of a soluble version of HeV-sG-V for safety, tolerability, and immunogenicity. The highest response rates were among vaccinees receiving two administrations of the 100 mcg vaccine candidate 28 days apart.
As of June 6, 2024, several Nipah Virus vaccine candidates were conducting clinical research, but no approved Nipah vaccines were available.
The World Health Organization says that Nipah was first discovered in Malaysia in the 1990s. This virus causes yearly outbreaks throughout South and Southeast Asia, with associated mortality rates of 40 to 75 %. Nipah infection is a zoonotic illness transmitted to people from animals such as bats.
The virus can also be transmitted through contaminated food or from person to person.
The WHO published a Technical Brief in early 2024 as an interim document to guide countries in planning for a Nipah virus event.
No cases of Nipah have been diagnosed in the U.S.

The U.S. Food and Drug Administration (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC) unanimously voted today to recommend that COVID-19 vaccines be updated to a monovalent JN.1-lineage composition for 2024-2025 and expressed a preference for the JN.1 strain.
As of June 5, 2024, Novavax Inc. stated in a press release that, pending authorization, it expects to be ready for the commercial delivery of a protein-based JN.1 COVID-19 vaccine in the U.S. in September 2024.
Novavax’s JN.1 COVID-19 vaccine has demonstrated broad cross-neutralizing antibodies for various JN.1 descendant viruses, including KP.2 and KP.3.
'We believe updating to the JN.1 lineage or JN.1, as recommended by the World Health Organization and the European Medicines Agency and as unanimously recommended by VRBPAC today, will provide the protection needed this fall against COVID-19,' wrote the company.
'Our most recent nonclinical data have demonstrated that our JN.1 vaccine candidate induces broad neutralization responses to JN.1 lineage viruses including those with the F456L mutation (e.g., JN.1.16), the R346T mutation (e.g., JN.1.13.1), to “FLiRT” variants that contain both mutations such as KP.2, currently the most common circulating variant in the U.S., and to “FLuQE” variants that are increasing in circulation (e.g., KP.3).'
'Our JN.1 vaccine candidate also produces conserved polyfunctional, Th1-biased CD4+ T cell responses to a range of JN.1 lineage variants, including those containing the F456L, R346T, and FLiRT mutations (e.g., KP.2).'
'These responses indicate that our vaccine technology induces broadly neutralizing responses against multiple variant strains, including circulating forward drift variants.'
Based on data presented by vaccine manufacturers today, the VRBPAC acknowledged the advantages of a JN.1 vaccine in providing broad protection against circulating and future strains and the need to minimize confusion in making public health recommendations.
As of June 2024, Novavax's vaccine is the only protein-based, non-mRNA vaccine available in the U.S. Novavax vaccines have been offered by most pharmacies in the U.S.

According to Stat News reporting today, Finland is preparing to offer 20,000 vaccines to people at risk of exposure to an avian influenza strain spreading among farmed and wild animals.
On June 5, 2024, Andrew Joseph wrote Finland may become the first country to offer 'bird flu' vaccinations to people.
According to the European Medicines Agency (EMA), the Aflunov vaccine is authorized for use in the European Union (EU) during avian influenza outbreaks without a declared pandemic.
Aflunov contains a flu strain called A/turkey/Turkey/1/2005 (H5N1)-like strain (NIBRG-23) (clade 2.2.1).
Additionally, the EMA says four pandemic-preparedness influenza vaccines authorized in the EU can be rapidly modified to protect people in a pandemic situation.
The EMA's human medicines committee recommended at its February 2024 meeting Celldemic (zoonotic influenza vaccine (H5N1)(surface antigen, inactivated, adjuvanted, prepared in cell cultures)). This vaccine is intended for immunization during outbreaks of influenza coming from animals, including when public health authorities anticipate a possible pandemic.
The Incellipan (pandemic influenza vaccine (H5N1) is a pandemic preparedness vaccine intended for use only if a flu pandemic has been officially declared.
Previously, the European Commission (EC) signed a framework contract on July 28, 2022, for the joint procurement of GSK's Adjupanrix, a pandemic influenza vaccine. As a result, EC Member States can purchase up to 85 million vaccine doses, if necessary, during an influenza pandemic.
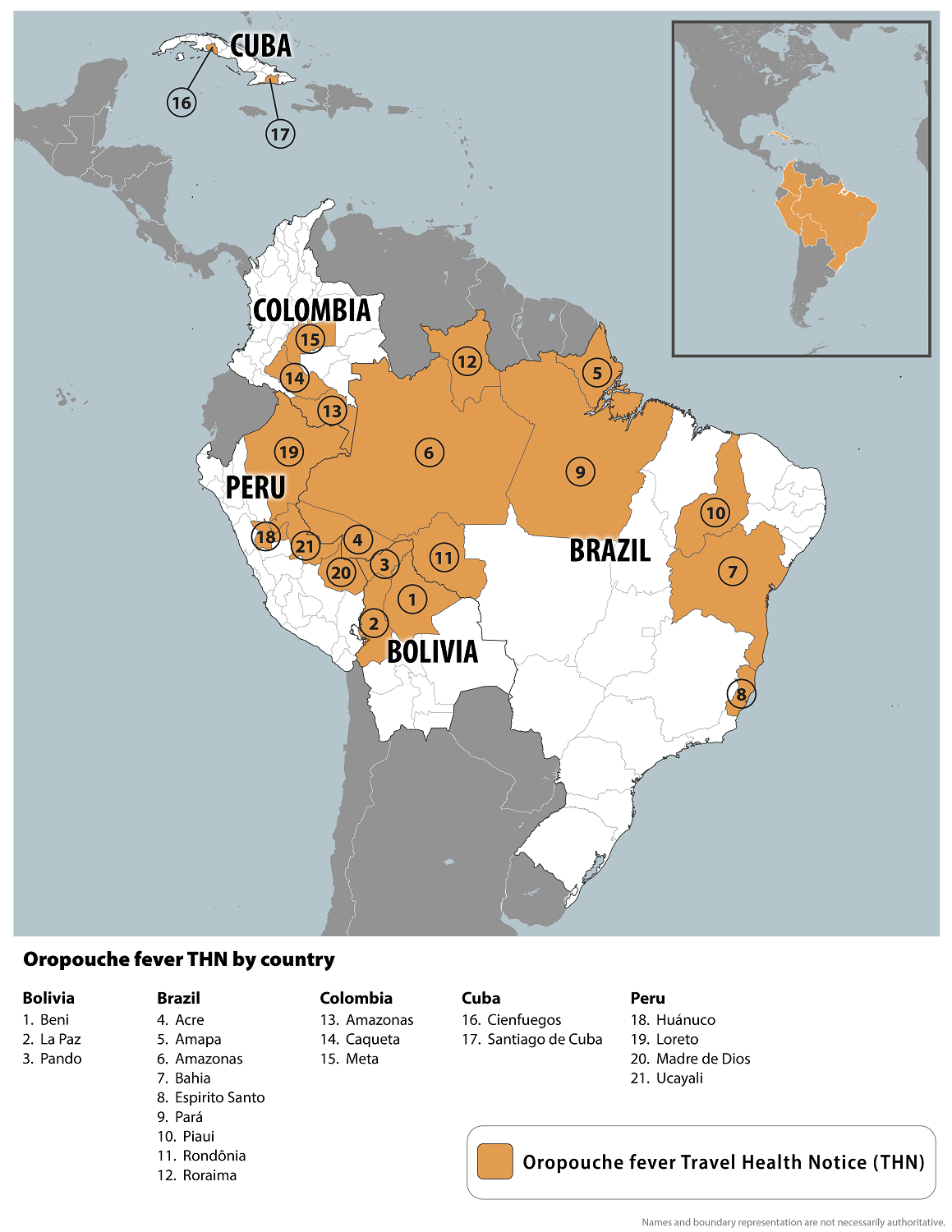
The U.S. Centers for Disease Control and Prevention (CDC) reported today that Oropouche fever outbreaks occur in 21 countries, including parts of Brazil, Bolivia, Colombia, Peru, Cuba, Ecuador, Panama, and Peru.
As of June 5, 2024, the CDC's Travel Health Advirosy says travelers should seek medical care if they develop high fever, headache, muscle aches, stiff joints, nausea, vomiting, chills, or sensitivity to light during or after travel.
Since it was first isolated in Trinidad and Tobago in 1955, more than half a million Oropouche cases and at least 30 major outbreaks have been reported. However, these data are difficult to quantify due to the lack of diagnosis.
In 2024, the Pan American Health Organization reported over 5,000 confirmed cases of Oropouche in four countries in the Region of the Americas.
This illness can occur in people of any age and is often mistaken for dengue, which is also impacting these populations.
The CDC says Oropouche fever is spread by the bite of infected midges (small flies) and mosquitoes.
Unfortunately, Oropouche infection treatment is supportive, as no specific medications or vaccines are available.
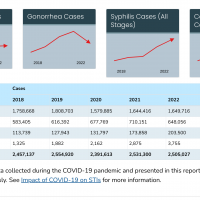
The U.S. Centers for Disease Control and Prevention (CDC) published clinical guidelines on counseling patients regarding doxycycline post-exposure prophylaxis (doxy PEP) for bacterial sexually transmitted infection (STI) prevention.
Doxycycline, a broad-spectrum tetracycline antimicrobial, is well absorbed and tolerated, with a half-life of approximately 12 hours.
As of June 4, 2024, the CDC says Doxy PEP has proven to reduce the risk of getting bacterial STIs, specifically syphilis, chlamydia, and gonorrhea.
The CDC recommends healthcare providers discuss doxy PEP with all gay, bisexual, and other men who have sex with men and transgender women with a history of at least one bacterial STI in the last 12 months. If offering doxy PEP, healthcare providers should prescribe self-administration of 200 mg of doxycycline within 72 hours of sex.
Laura Bachmann, MD, MPH, FIDSA, FACP, Acting Director, Division of STD Prevention, stated in a media release, 'Doxy PEP represents the first new STI prevention tool in decades, at a time when innovation in the nation's fight nation's STIs is desperately needed."
As of June 5, 2024, bacterial STI vaccine candidates are conducting clinical research.
Recently, the Bexsero® meningitis B vaccine (4CMenB) has shown some protection against gonorrhea. Studies have found that people who receive two doses of Bexsero maybe 33–40% less likely to be diagnosed with gonorrhea than those who don't.
In the U.K., meningococcal vaccination is now advised for certain people to prevent STIs.