Search API
Sysmex Astrego AB announced today that it was awarded the Longitude Prize for antimicrobial resistance for developing a rapid antimicrobial susceptibility test for urinary tract infections (UTIs).
Sysmex Astrego received the $10.2 million award to incentivize the development of transformative point-of-care tests that will improve antibiotic treatment decisions.
Using a 400 microlitre urine sample on a smartphone-sized cartridge, the PA-100 AST System test can identify the presence of bacterial infections such as UTIs in just 15 minutes.
The goal is to replace the 2-3 day lab test process.
"Winning the Longitude Prize is the first true and biggest recognition that what we have been doing all these years was for a very important global cause," Ozden Baltekin, PhD, Sysmex Astrego director of program management, said in a press release on June 12, 2024.
Sysmex Astrego launched the PA-100 AST System in Europe in 2023 and intends to accelerate global expansion efforts.
UTIs are the most common bacterial infection, and around 50-60% of women develop one in their lifetime.
As of June 13, 2024, a UTI vaccine is available in certain countries, and new therapies are conducting late-stage development.

Gavi, the Vaccine Alliance, today announced support for human rabies vaccines for post-exposure prophylaxis (PEP) as part of routine immunization.
On June 13, 2024, Gavi stated eligible countries are receiving guidance on how to access these vaccines under Gavi’s cofinancing policy. The first round of applications will be accepted by mid-July 2024. Ninety-five percent of human rabies deaths occur in Africa and Asia.
“This commitment from Gavi is crucial and will expedite efforts to halt human fatalities caused by dog-mediated rabies,” said Dr Jérôme Salomon, Assistant Director-General for Universal Health Coverage, Communicable and Noncommunicable Diseases at WHO, in a press release.
“WHO will provide technical assistance to countries, not only to support their funding applications to Gavi but to draw up comprehensive plans of action that can deliver real progress towards the Zero by 30 goal.”
This development complements the ongoing global efforts of the Zero by 30 campaign, led by United Against Rabies partners, including the Food and Agriculture Organization, the World Health Organization, and the World Organisation for Animal Health, to eliminate dog-mediated human rabies by 2030.
In the United States, the Centers for Disease Control and Prevention reports bats, not dogs, are the leading source of rabies cases.

With 52 countries reporting measles outbreaks over the past year, one international gateway county recently reported a surprising number of measles cases in 2024.
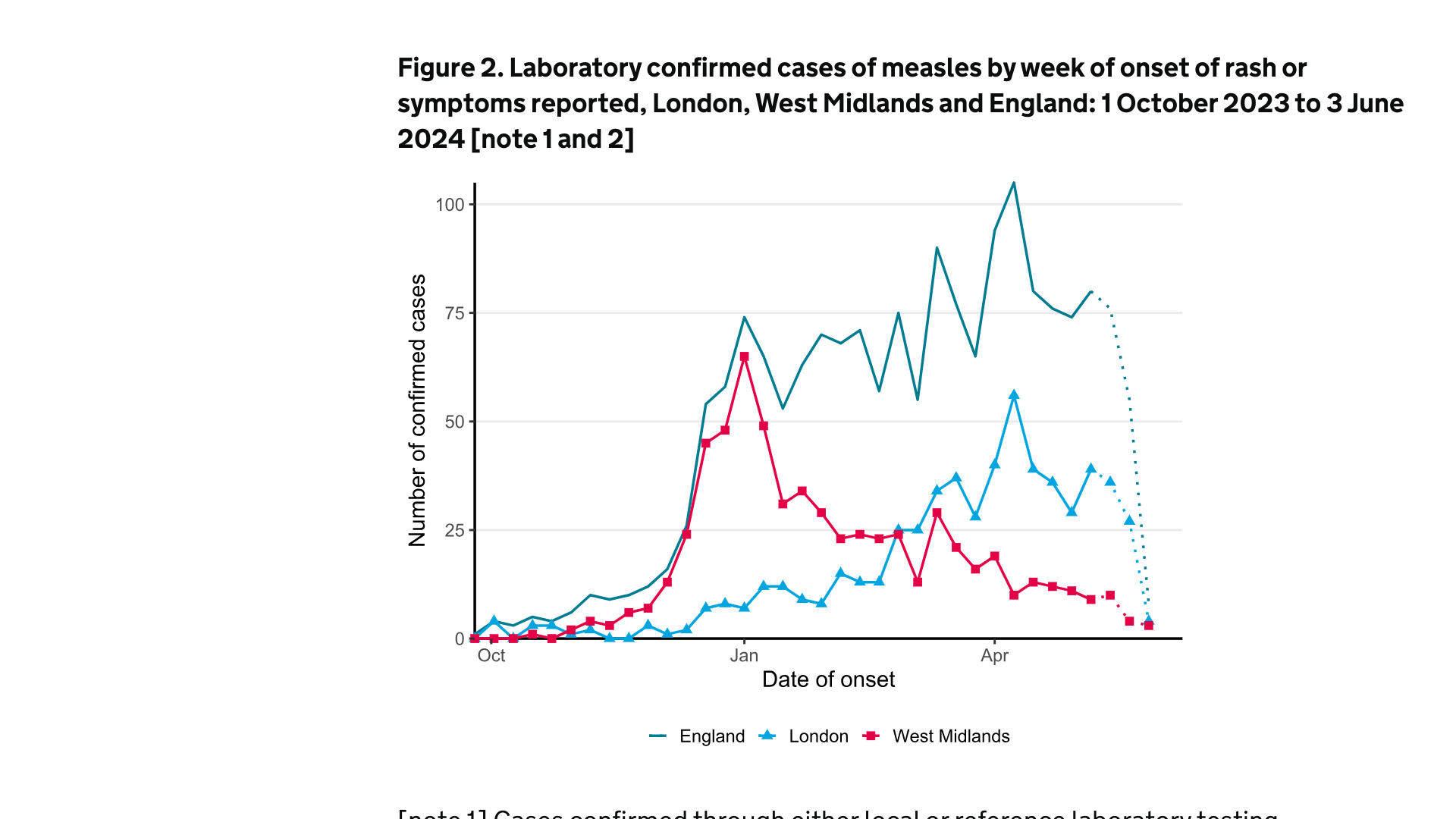
As of June 3, 2024, the U.K. Health Security Agency (UKHSA) reported 1,749 laboratory-confirmed measles cases had been reported in England since October 2023.
England's London and West Midlands areas continued leading the measles outbreak over the last month when 219 confirmed measles cases were confirmed.
The most significant number of cases were in London (106), followed by the West Midlands, East of England, East Midlands, and the North East.
Dr.Vanessa Saliba, UKHSA Consultant Epidemiologist, commented in a May 2024 press release, "The number of measles cases is rising across the country, with a particular increase in London in recent weeks."
"We know some communities in London have very low MMR vaccination rates."
Compared with England, measles outbreaks in the United States appear under control.
As of June 7, 2024, the U.S. CDC reported 151 measles cases in 22 jurisdictions, primarily in Chicago. Most of the Illinois patients were unvaccinated travelers.
The CDC encourages international travelers to speak with a healthcare provider about their measles immunity and travel vaccine options.
The Democratic Republic of the Congo (DRC) is having its largest surge of mpox cases ever recorded, says the U.S. CDC. Since January 2023, the DRC has reported more than 20,000 suspected mpox cases, and about 1,000 deaths have been reported.
To notify international travelers of their potential mpox risk, the CDC reissued a Travel Health Advisory on June 10, 2024.
Mpox is a disease caused by infection with the monkeypox virus. It is divided into two Clades. Clade IIb is responsible for the global outbreak that began in May 2022, while Clade 1 is causing the Mpox outbreak in the DRC.
There have been no cases of the type of mpox spreading in DRC reported in the United States, says the CDC. The risk to the general public in the U.S. from this type of mpox is very low.
In 2023, the CDC published a Health Advisory stating that mpox Vaccines are expected to be effective for both Clade I and Clade II infections. However, as the European CDC recently reported, real-world data regarding the effectiveness of the JYNNEOS vaccine against Clade 1 is lacking.

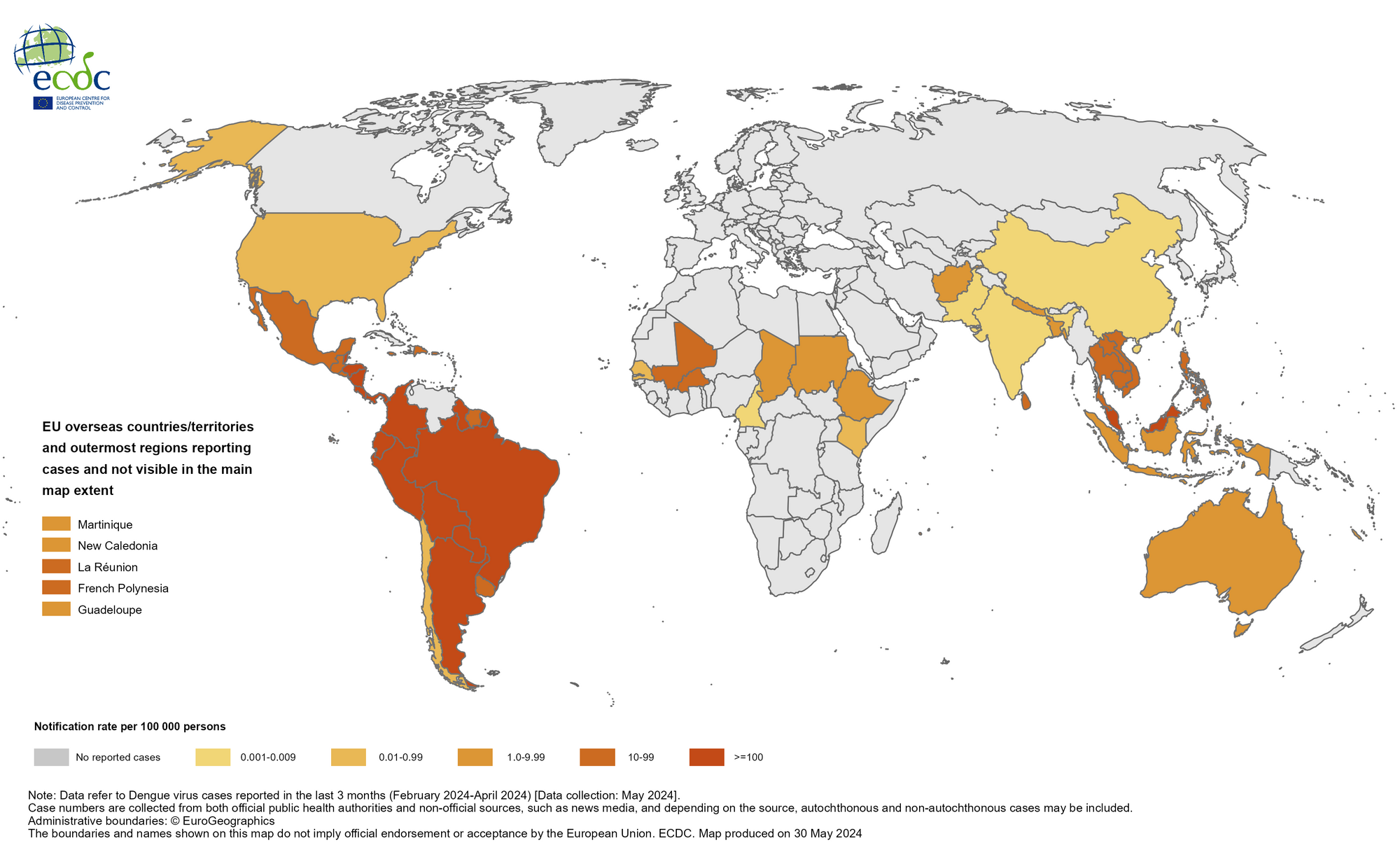
With most of the dengue outbreak risks revealed in The Americas in 2024, cases of this invasive mosquito-spreading disease are also impacting Europe.
According to the European Centre for Disease Prevention and Control (ECDC) reporting on June 11, 2024, there were 130 locally acquired dengue cases in the European Union, plus Iceland, Liechtenstein, and Norway, compared to 71 in 2022.
According to ECDC data, this was a "significant increase" in dengue reports from the 2010-2021 period, when the number for the entire period was 73.
Imported dengue cases have also increased from 1,572 cases in 2022 to about 4,900 in 2023, "the highest number" since 2008.
"Europe is already seeing how climate change is creating more favorable conditions for invasive mosquitos to spread into previously unaffected areas and infect more people with diseases such as dengue. Increased international travel from dengue-endemic countries will also increase the risk of imported cases, and inevitably also the risk of local outbreaks," says Andrea Ammon, ECDC Director.
The ECDC says Aedes albopictus, known for transmitting dengue, chikungunya, and Zika viruses, is spreading further north, east, and west in Europe and now has self-sustaining populations across 13 EU/EEA countries.
Aedes aegypti, a vector of yellow fever, dengue, chikungunya, and Zika viruses, recently established itself in Cyprus. Its potential for establishment in other parts of Europe is concerning due to its significant ability to transmit pathogens and its preference for biting humans.
The Culex pipiens mosquito, responsible for the spread of West Nile virus, is native to Europe and is present throughout the EU/EEA.
From a prevention perspective, the second-generation Qdenga vaccine is now offered in many EU countries.
CSL Seqirus today announced it was selected by the Health Emergency Preparedness and Response Authority (HERA) to provide 665,000 pre-pandemic vaccine doses for fifteen EU and EEA Member States and the "Union Civil Protection Mechanism.".
The 4-year contract includes an option for participating authorities to purchase up to an additional 40 million doses of the egg-based, non-mRNA pre-pandemic vaccine over the contract duration.
Announced on June 11, 2024, this European Commission (EC) acquisition of pre-pandemic (zoonotic) vaccine will create a stockpile of vaccines available to support the EC’s outbreak and pre-pandemic response.
According to media reporting on June 5, 2024, Finland became the first EU country to offer avian influenza vaccinations to people this year.
Four influenza pandemics have occurred over the past century, with the 1918 pandemic being the most severe in recent history, with an estimated mortality of up to 50 million people worldwide.
Raja Rajaram, CSL Seqirus, Head of Global Medical Strategy, commented in a press release, “This agreement will help in Europe’s resolve to maintain robust preparedness and rapid response capabilities for this potential threat.”
Under the terms of the agreement, CSL Seqirus will deliver pre-pandemic vaccines that are well-matched to the H5 of the currently circulating H5N1 strain.
The risk of influenza-associated morbidity and mortality is greater with pandemic influenza than with seasonal influenza because there is likely to be little or no pre-existing immunity to the novel virus in the human population. The timing and severity of pandemic influenza (bird flu) is unpredictable.
In Europe, various pandemic vaccines have already been approved.
In the U.S., the Food and Drug Administration has already approved CSL Sequirus's AUDENZ™, an inactivated vaccine for active immunization to prevent disease caused by the influenza A virus H5N1 subtype.
As of June 11, 2024, the U.S. CDC says annual flu shots are not designed to protect people from pandemic influenza.




