Search API
Novavax, Inc. today announced that it has filed for a type II variation of existing Marketing Authorization with the European Medicines Agency (EMA) for its JN.1 COVID-19 vaccine (NVX-CoV2705) for individuals aged 12 and older.
The submission follows guidance from EMA and the World Health Organization to target the JN.1 lineage for the Fall 2024 season.
Novavax's non-mRNA JN.1 COVID-19 vaccine targets the "parent strain" of KP.2 and KP.3.2
NVX-CoV2705 is an updated version of Novavax's authorized COVID-19 vaccine (NVX-CoV2373).
"Novavax is working closely with European markets seeking to offer a protein-based alternative to mRNA this fall for COVID-19 vaccination," said John C. Jacobs, President and Chief Executive Officer, Novavax, in a press release on June 24, 2024.
"Our updated COVID-19 vaccine is active against current circulating (SARS-CoV-2) strains, including KP.2 and KP.3."
Nonclinical data have demonstrated that Novavax's JN.1 COVID-19 vaccine induces broad neutralization responses to JN.1 lineage viruses, including those containing the F456L and R346T mutations, and to "FLiRT" and "FLuQE" variants.
Novavax's vaccine also produces conserved polyfunctional, Th1-biased CD4+ T cell responses to a range of JN.1 lineage variants.
Novavax confirmed it intends to have its JN.1 COVID-19 vaccine in unit-dose vials available for immediate release in the European Union after approval.
Novavax has also filed with the U.S. FDA and is working with other regulatory authorities globally to authorize or approve its JN.1 COVID-19 vaccine.

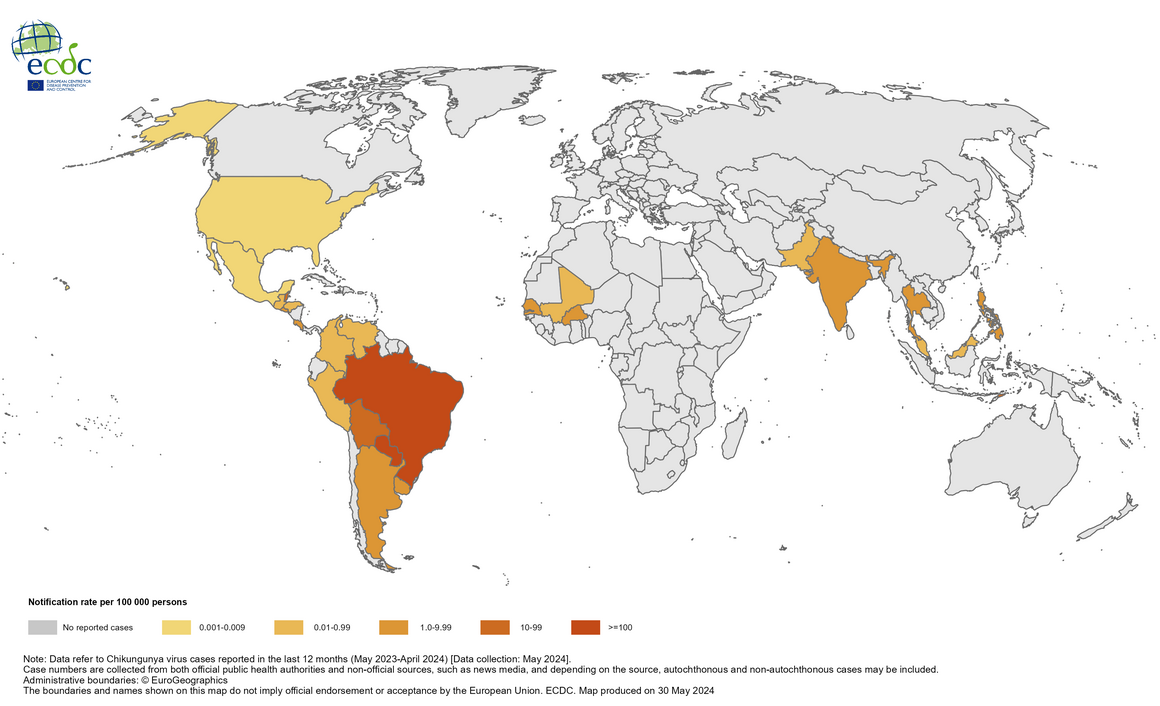
Since 2004, the Chikungunya virus (CHIKV) has caused large-scale outbreaks worldwide and has recently been identified in over 110 countries.
According to news from Valneva SE, a second country has approved IXCHIQ®, the world's first licensed chikungunya vaccine, to address this unmet medical need.
Valneva announced on June 24, 2024, that Health Canada had approved the IXCHIQ vaccine to prevent this mosquito-transmitted disease for adults.
This new approval follows the U.S. Food and Drug Administration decision in November 2023. The European Medicines Agency recently recommended marketing authorization for IXCHIQ in Europe, and a formal decision is expected in the third quarter of 2024.
On June 26, 2024, the U.S. CDC's vaccine committee plans to discuss how this innovative vaccine can be offered in the United States.
Chikungunya's economic burden is expected to increase as the mosquito vectors transmitting CHIKV spread geographically. As such, the World Health Organization recently highlighted chikungunya outbreaks as a significant public health problem.
According to the U.S. Centers for Disease Control and Prevention (CDC), new data indicates a continued reduction in influenza-associated pediatric deaths.
During Week #24 of 2024, three influenza-associated pediatric deaths were reported during the 2023-2024 season.
One of the recent deaths was associated with an influenza A(H3) virus, the other with an influenza B/Victoria virus, and the third with an influenza B virus.
As of June 21, 2024, the CDC had reported 178 influenza-associated pediatric deaths during the 2023-2024 season.
This amount is lower than last flu season, when 185 children died from influenza infections.
Moreover, it is lower than in 2019-2020, when 199 influenza-associated pediatric deaths were reported.
CDC recommends that everyone six months and older get an annual flu vaccine as long as flu activity continues. Flu shots remain available at most pharmacies in the U.S.
However, current CDC data indicates an unusual correlation between pediatric deaths and vaccinations. While flu-related deaths are slowing, so are the number of influenza vaccinations.
During the current flu season, about 158 million influenza vaccines were distributed in the U.S.
The CDC previously reported that 173 million influenza vaccines were distributed during the 2022-2023 flu season and 194 million during the 201-2022 season.

As the threat of antibiotic resistance grows, researchers are developing ways to prevent recurrent and chronic urinary tract infections (UTIs) without using antibiotics, wrote Carissa Wong on May 2, 2024.
An article published in the journal Nature says the latest approaches include an oral spray vaccine.
In clinical trials, the pineapple-flavored Uromune™ (MV140) prevented recurrent UTIs in participants for up to nine years. The polyvalent bacterial whole-cell-based sublingual vaccine is sprayed under the tongue daily for three months.
Unfortunately, Uromune is currently unavailable in Canada or the United States. But it is offered in various countries.
Furthermore, scientists are also testing safer ways to treat UTI infections with antibiotics, which often cause side effects.
The anti-infective candidate RECCE® 327 (R327) was recently added to the World Health Organization's report on Antibacterial Agents in Clinical Development and Preclinical Development.
The U.S. CDC says UTIs are common infections caused by bacteria, often from the skin or rectum, entering the urethra and infecting the urinary tract.
UTIs are more common in females because their urethras are shorter and closer to the rectum. This makes it easier for bacteria to enter the urinary tract, says the CDC.
However, about 10% of men will also experience a UTI during their life.
Younger children may not be able to tell you about their UTI symptoms. While fever is the most common sign of a UTI in infants and toddlers, most children with fever do not have a UTI.
Access to the complete Nature article is at this link.

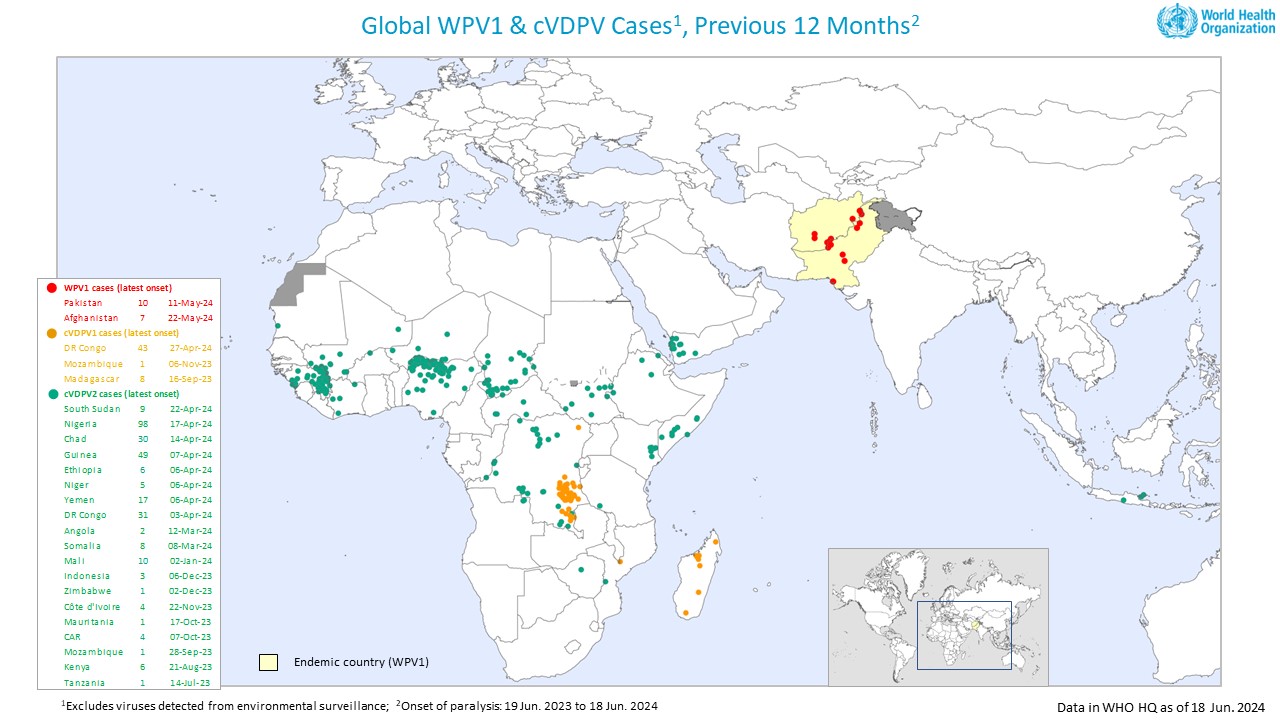
The Global Polio Eradication Initiative (GPEI) released its weekly update, which indicated that three countries reported new polio cases as the poliovirus continues to spread in 2024.
On June 19, 2024, the GPEI reported that Afghanistan confirmed one wild poliovirus type 1 (WPV1), bringing the total number of cases in 2024 to six.
Nigeria reported three cases of circulating vaccine-derived poliovirus type 2 (cVDPV2), bringing the total for the year to 30.
And South Sudan reported its sixth cVDPV2 case of the year.
The U.S. CDC stated on May 23, 2024, that before traveling to any of these 34 destinations, adults who had previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of the polio vaccine.
In the U.S., the IPV vaccine is generally available at pharmacies and travel clinics. In Africa, the nOPV2 vaccine has become standard in most countries during 2024.
The nOPV2 has been 'triple-locked' using genetic engineering to prevent it from producing a gut reaction.
As a result, the GPEI reports that nOPV2 is more genetically stable than approved oral polio vaccines, with a lower risk of reversion to neurovirulence and less likely to mutate and cause paralysis.
With the expanding geographic range of disease-carrying mosquitoes, more people than ever are being infected with dengue fever this year.
In the Region of the Americas, the number of dengue cases during the first half of 2024 exceeded the maximum number compared to all previously recorded years.
According to the Pan American Health Organization's (PAHO) latest report, 43 countries and territories in the Region of the Americas have reported 9,386,082 cases of dengue.
However, in 2023, only 4,617,108 dengue cases were reported by the PAHO.
In the United States, the Centers for Disease Control and Prevention (CDC) reported that as of mid-June 2024, 43 jurisdictions, led by Florida, New York, and Puerto Rico, reported 1,984 dengue cases.
The CDC says dengue is endemic in the U.S. territories of Puerto Rico, American Samoa, the U.S. Virgin Islands, the Federated States of Micronesia, the Republic of Marshall Islands, and the Republic of Palau.
To alert international travelers of the risk of dengue infection, the CDC reissued a Global Travel Health Notice on June 20, 2024, regarding outbreaks in 30 countries.
To prevent serious health issues, the WHO has prequalified two dengue vaccines.
On May 15, 2024, the WHO announced that it prequalified the Takeda-developed QDENGA® (TAK-003) vaccine. This dengue vaccine does not require pre-admission testing.