Search API
According to the U.S. Centers for Disease Control and Prevention (CDC), new data indicates a continued reduction in influenza-associated pediatric deaths.
During Week #24 of 2024, three influenza-associated pediatric deaths were reported during the 2023-2024 season.
One of the recent deaths was associated with an influenza A(H3) virus, the other with an influenza B/Victoria virus, and the third with an influenza B virus.
As of June 21, 2024, the CDC had reported 178 influenza-associated pediatric deaths during the 2023-2024 season.
This amount is lower than last flu season, when 185 children died from influenza infections.
Moreover, it is lower than in 2019-2020, when 199 influenza-associated pediatric deaths were reported.
CDC recommends that everyone six months and older get an annual flu vaccine as long as flu activity continues. Flu shots remain available at most pharmacies in the U.S.
However, current CDC data indicates an unusual correlation between pediatric deaths and vaccinations. While flu-related deaths are slowing, so are the number of influenza vaccinations.
During the current flu season, about 158 million influenza vaccines were distributed in the U.S.
The CDC previously reported that 173 million influenza vaccines were distributed during the 2022-2023 flu season and 194 million during the 201-2022 season.

As the threat of antibiotic resistance grows, researchers are developing ways to prevent recurrent and chronic urinary tract infections (UTIs) without using antibiotics, wrote Carissa Wong on May 2, 2024.
An article published in the journal Nature says the latest approaches include an oral spray vaccine.
In clinical trials, the pineapple-flavored Uromune™ (MV140) prevented recurrent UTIs in participants for up to nine years. The polyvalent bacterial whole-cell-based sublingual vaccine is sprayed under the tongue daily for three months.
Unfortunately, Uromune is currently unavailable in Canada or the United States. But it is offered in various countries.
Furthermore, scientists are also testing safer ways to treat UTI infections with antibiotics, which often cause side effects.
The anti-infective candidate RECCE® 327 (R327) was recently added to the World Health Organization's report on Antibacterial Agents in Clinical Development and Preclinical Development.
The U.S. CDC says UTIs are common infections caused by bacteria, often from the skin or rectum, entering the urethra and infecting the urinary tract.
UTIs are more common in females because their urethras are shorter and closer to the rectum. This makes it easier for bacteria to enter the urinary tract, says the CDC.
However, about 10% of men will also experience a UTI during their life.
Younger children may not be able to tell you about their UTI symptoms. While fever is the most common sign of a UTI in infants and toddlers, most children with fever do not have a UTI.
Access to the complete Nature article is at this link.

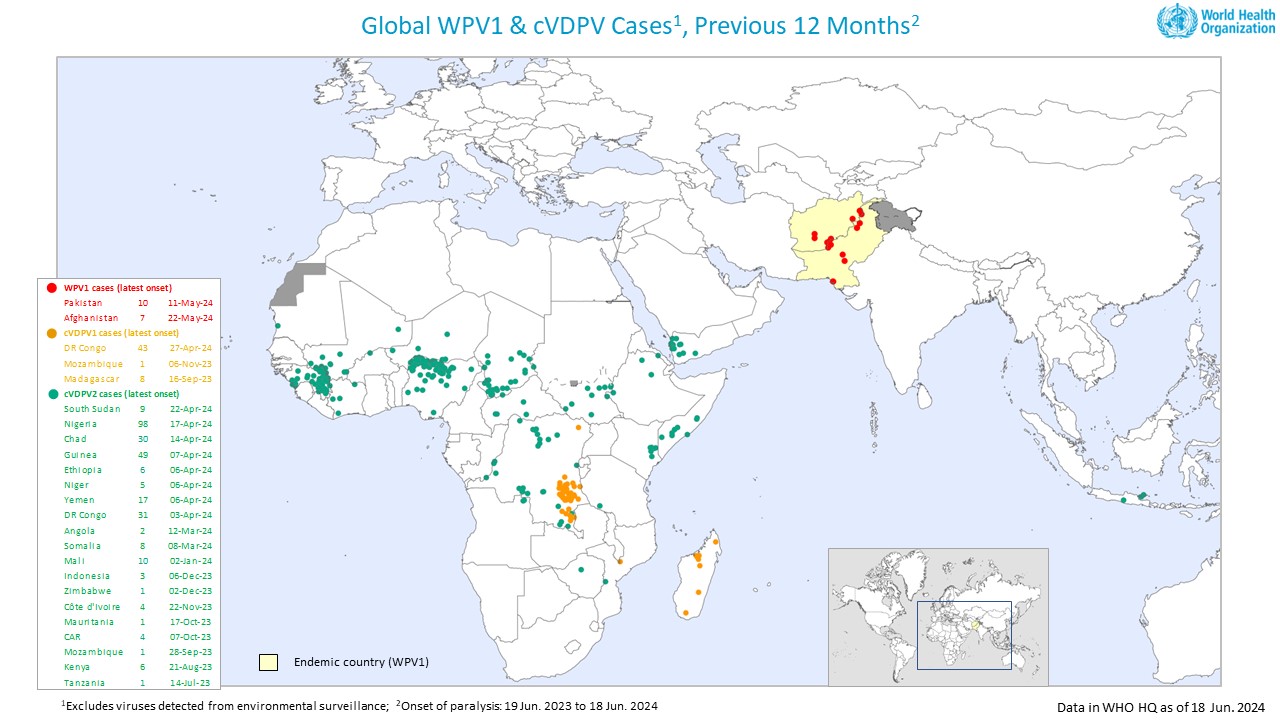
The Global Polio Eradication Initiative (GPEI) released its weekly update, which indicated that three countries reported new polio cases as the poliovirus continues to spread in 2024.
On June 19, 2024, the GPEI reported that Afghanistan confirmed one wild poliovirus type 1 (WPV1), bringing the total number of cases in 2024 to six.
Nigeria reported three cases of circulating vaccine-derived poliovirus type 2 (cVDPV2), bringing the total for the year to 30.
And South Sudan reported its sixth cVDPV2 case of the year.
The U.S. CDC stated on May 23, 2024, that before traveling to any of these 34 destinations, adults who had previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of the polio vaccine.
In the U.S., the IPV vaccine is generally available at pharmacies and travel clinics. In Africa, the nOPV2 vaccine has become standard in most countries during 2024.
The nOPV2 has been 'triple-locked' using genetic engineering to prevent it from producing a gut reaction.
As a result, the GPEI reports that nOPV2 is more genetically stable than approved oral polio vaccines, with a lower risk of reversion to neurovirulence and less likely to mutate and cause paralysis.
With the expanding geographic range of disease-carrying mosquitoes, more people than ever are being infected with dengue fever this year.
In the Region of the Americas, the number of dengue cases during the first half of 2024 exceeded the maximum number compared to all previously recorded years.
According to the Pan American Health Organization's (PAHO) latest report, 43 countries and territories in the Region of the Americas have reported 9,386,082 cases of dengue.
However, in 2023, only 4,617,108 dengue cases were reported by the PAHO.
In the United States, the Centers for Disease Control and Prevention (CDC) reported that as of mid-June 2024, 43 jurisdictions, led by Florida, New York, and Puerto Rico, reported 1,984 dengue cases.
The CDC says dengue is endemic in the U.S. territories of Puerto Rico, American Samoa, the U.S. Virgin Islands, the Federated States of Micronesia, the Republic of Marshall Islands, and the Republic of Palau.
To alert international travelers of the risk of dengue infection, the CDC reissued a Global Travel Health Notice on June 20, 2024, regarding outbreaks in 30 countries.
To prevent serious health issues, the WHO has prequalified two dengue vaccines.
On May 15, 2024, the WHO announced that it prequalified the Takeda-developed QDENGA® (TAK-003) vaccine. This dengue vaccine does not require pre-admission testing.

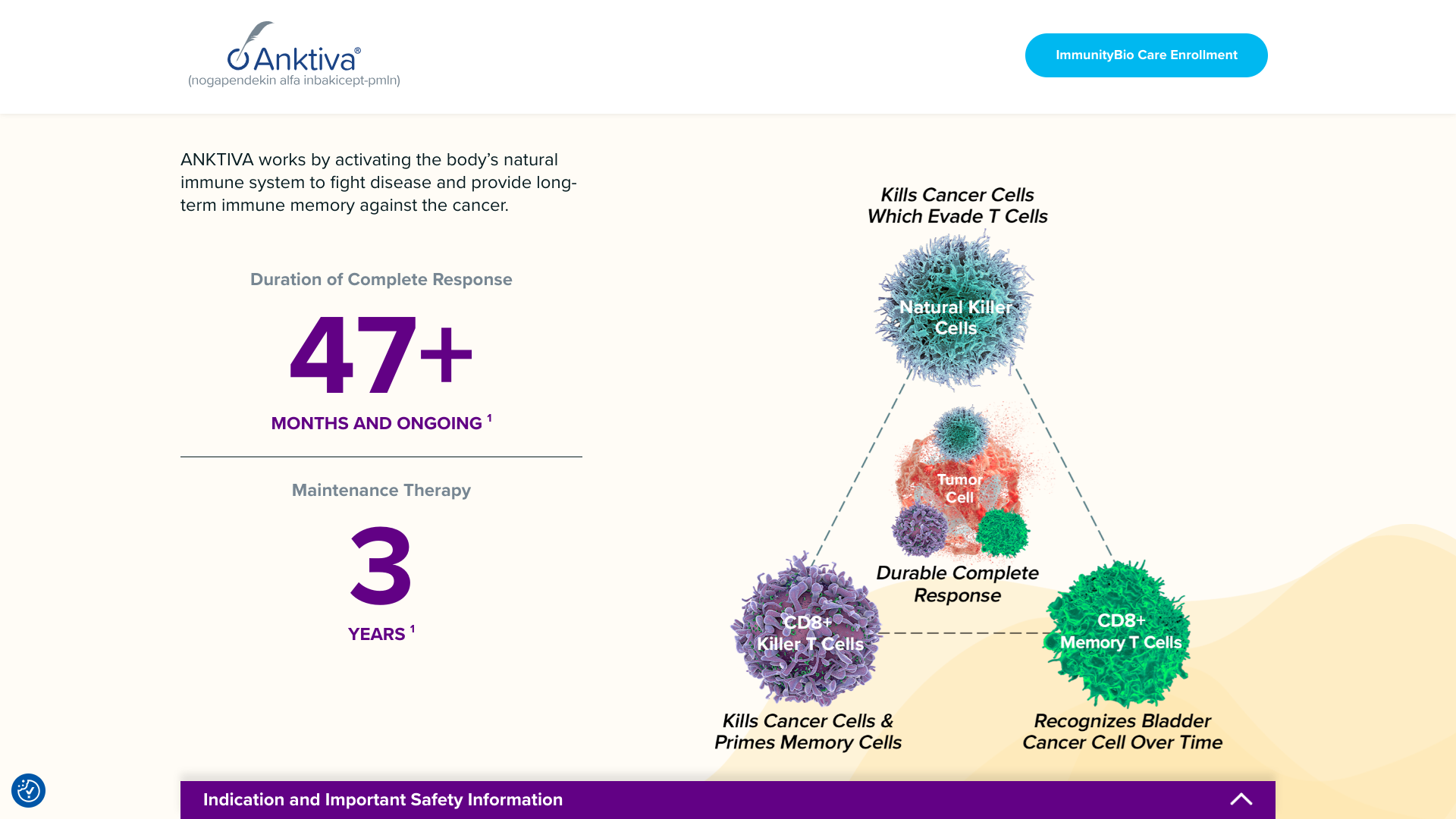
ImmunityBio, Inc. today announced the initial treatment of multiple patients in the U.S. to receive therapy with ANKTIVA®, the company's recently approved immunotherapy for Bacillus Calmette-Guérin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC) carcinoma in situ.
The first bladder cancer patients to receive commercial doses are located throughout the U.S., and several are being treated by community urologists, as the therapy does not require any special handling or equipment that would limit its use to specialty medical centers.
ANKTIVA (nogapendekin alfa inbakicept-pmln) was approved by the U.S. Food and Drug Administration (FDA) on April 22, 2024, for the treatment of patients with BCG-unresponsive NMIBC CIS with or without papillary tumors.
The intravesical therapy employs a combination of ANKTIVA, an IL-15 agonist, in combination with the BCG vaccine.
The combination is the first FDA-approved immunotherapy in NMIBC that functions by activating the body’s NK and killer T-cell immune system to attack tumor cells while simultaneously activating memory T cells, leading to a prolonged duration of complete response exceeding 47 months for some patients.
“In addition to its unique mechanism of action, ANKTIVA can be readily administered by urologists in their own offices and clinics, enabling more patients to receive it in familiar settings from their own providers,” said Richard Adcock, President and CEO of ImmunityBio, in a press release on June 20, 2024.
“We look forward to ANKTIVA reaching more and more eligible NMIBC patients and for our science to deliver even more therapies from our pipeline.”
In May 2024, ImmunityBio announced it had drug substance sufficient for 170,000 doses of ANKTIVA for commercial and clinical trial use.
In the U.S., the American Cancer Society estimates there will be 83,190 new cases and 16,840 deaths from bladder cancer in 2024.
A position paper published online in the Annals of Allergy, Asthma & Immunology reviewed the safety of administering live vaccines to people currently treated with Dupilumab®, a biologic therapy.
Dupilumab is a monoclonal antibody that targets the interleukin (IL)-4 receptor alpha subunit, thus blocking the effects of IL-4 and IL-13. It has shown efficacy in treating various conditions, including asthma, atopic dermatitis, eosinophilic esophagitis, and others.
Because Dupilumab is now approved for use in patients from six months of age for the treatment of atopic dermatitis, a reported contraindication is now posing a clinical dilemma for patients and clinicians.
After a systematic review of available data, this panel concluded on June 5, 2024, that stopping treatment with Dupilumab is unnecessary to administer live vaccines.
However, the panel recommends that doctors and patients/parents engage in shared decision-making to determine the timing and necessity of administering live vaccines to individuals being treated with Dupilumab or whether treatment should be paused.
As a clinical resource, board-certified allergists/immunologists have special training in managing and treating people with asthma or allergies who may benefit from this medication.
According to MedlinePlus, there are five types of vaccines are currently available, including live virus vaccines. These vaccines use the weakened (attenuated) form of the targeted virus. The MMR and the varicella (chickenpox) vaccines are examples.

Vaxxinity, Inc. announced today that the journal Nature Medicine has published groundbreaking exploratory data from the Company's Phase 1 clinical trial of UB-312 in patients with Parkinson's disease (PD).
The Phase 1 successfully met its primary outcome measures, demonstrating that UB-312 was generally well-tolerated and induced anti-aggregated α-synuclein (αSyn) antibody responses in healthy volunteers and PD patients. Specifically, 12 of 13 PD patients who completed dosing developed anti-αSyn antibodies.
And UB-312-induced antibodies significantly decreased levels of αSyn, a key pathology in PD and other synucleinopathies. This suggests that UB-312 can help to eliminate the buildup of harmful, toxic forms of the protein αSyn in the brain.
Furthermore, patients with detectable UB-312-induced antibodies in cerebrospinal fluid exhibited significant improvement in motor experiences of daily living as measured by the MDS-UPDRS Part II, a commonly accepted clinical scale.
This announcement marks a potentially significant milestone in pursuing innovative PD care.
"UB-312 has the potential to become an important and potent disease-modifying therapy for Parkinson's disease. It would be truly amazing if we could vaccinate people against Parkinson's disease in the future!" said Professor Geert Jan Groeneveld, neurologist and principal investigator of the Phase 1 clinical trial performed at the Centre for Human Drug Research in Leiden, the Netherlands, in a press release on June 20, 2024.
Parkinson's disease, a progressive neurodegenerative condition, currently lacks an approved disease-modifying treatment. Alpha-synuclein, a key protein in PD pathology, forms aggregates known as Lewy bodies that contribute to neuronal degeneration.
This active immunotherapy medicines research was funded by the Michael J. Fox Foundation, assessing target engagement in collaboration with the Mayo Clinic and UTHealth Houston in Texas.



