Search API
Bavarian Nordic A/S today announced the submission of a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) seeking approval of the Company’s vaccine candidate for immunization to prevent disease caused by chikungunya virus infection in individuals 12 years and older.
CHIKV VLP is an adjuvanted VLP-based, single-dose vaccine candidate for active immunization to prevent disease caused by CHIKV infection.
The MAA application was granted accelerated assessment by the Committee for Medicinal Products for Human Use in February 2024, supporting the potential approval of the vaccine by the European Commission in the first half of 2025.
In the past 20 years, the chikungunya virus has emerged in several previously non-endemic regions in Asia, Africa, southern Europe, and the Americas, often causing large, unpredictable outbreaks.
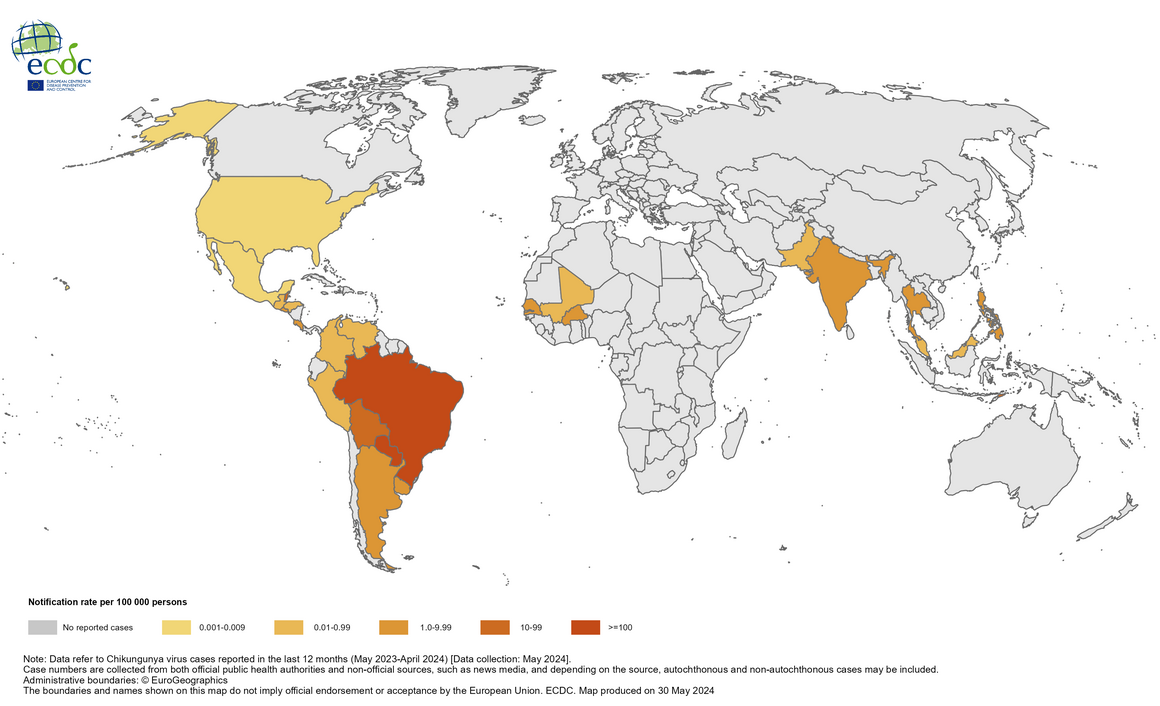
The ECDC says Chikungunya is not endemic in mainland Europe, and most cases are travelers infected outside of the mainland European Union/European Economic Area.
“Our CHIKV VLP vaccine is designed for ease of use in individuals 12 years of age and older at risk of chikungunya virus and represents an important contribution to the development of preventative solutions against this debilitating disease,” said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release on June 26, 2024.
The MAA submission includes results from two phase 3 clinical trials in more than 3,600 healthy individuals 12 years and older. The results showed that the CHIKV VLP vaccine was highly immunogenic, as demonstrated by the strong induction of Chikungunya neutralizing antibodies 21 days after vaccination, with antibody titers equal to or above the threshold agreed with authorities as a marker of seroprotection in the majority of individuals. The CHIKV VLP vaccine was well-tolerated across both studies, and vaccine-related adverse events were mainly mild or moderate in nature.
ILiAD Biotechnologies, LLC announced the selection of Emmes Group to conduct upcoming Phase III studies of its lead pertussis vaccine candidate, BPZE1.
As of June 24, 2024, ILiAD and Emmes Group are working to finalize the definitive agreement.
Multiple Phase III studies are expected to be conducted in North America, Central and South America, the U.K., and other global clinical sites.
BPZE1 is the leading next-generation pertussis vaccine designed to induce comprehensive and durable protection against B. pertussis infection (colonization) and disease (whooping cough). This vaccine is being developed to block B. pertussis from colonizing the nasal passages of adults and children, to protect adults and children from whooping cough, and to potentially prevent transmission, including transmission to infants.
"We are honored and pleased that ILiAD has selected Emmes Group as its partner to continue the clinical development of BPZE1. We look forward to working closely with ILiAD's clinical development team on this promising new vaccine, which could significantly reduce the transmissibility and incidence of B. pertussis, particularly in vulnerable populations," said Sastry Chilukuri, Chief Executive Officer of Emmes Group, in a press release.
While ILiAD is currently focused on developing a vaccine to protect adults and children and indirectly protect vulnerable infants, future development aims to immunize neonates directly. BPZE1 was developed at the Institut Pasteur de Lille (France) in the lab of Camille Locht, PhD and Nathalie Mielcarek, PhD.
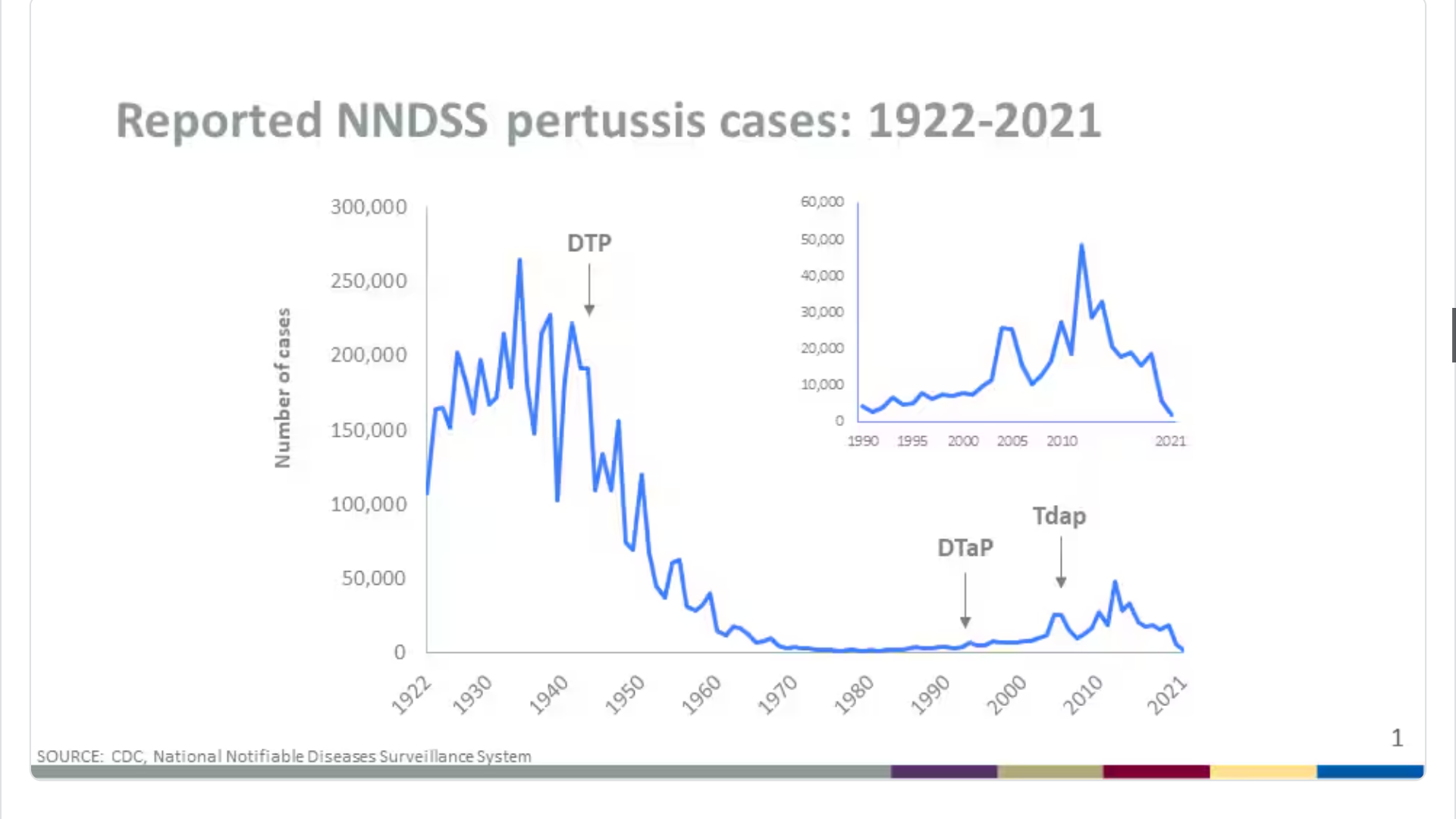
According to the U.S. CDC, reported pertussis cases in 2024 increased across the U.S., indicating a return to more typical trends. Preliminary data show that more than three times as many cases have been reported to date in 2024 compared to the same time in 2023.
Osivax today announced that it had completed enrollment in its Phase 1 clinical trial with OVX033, the company’s broad-spectrum vaccine candidate against sarbecoviruses.
OVX033 is a first-in-class coronavirus vaccine candidate that targets the nucleocapsid (N), a highly conserved internal antigen. Osivax is creating a revolutionary universal sarbecovirus vaccine that will uniquely empower both the B-cell immune response and the T-cell immune response.
Unlike surface antigens such as Spike (S), N is much less likely to mutate, providing a broader and more universal immune response to induce broad-spectrum protection against all current and future variants of the SARS-CoV-2 coronavirus and against future pandemic coronavirus strains.
“Sarbecoviruses remain a threat as evidenced by the (recent) pandemic, which continues to have long-term consequences for global health. By completing enrollment for our Phase 1 trial with OVX033, we are taking a significant step forward in addressing the need for a broad-spectrum vaccine to protect against these rapidly mutating viruses,” said Dr. Nicola Groth, CMO of Osivax, in a press release.
Osivax’s oligoDOMTM technology enables the design and production of a recombinant version of the nucleocapsid, which self-assembles into a nanoparticle and thus triggers powerful T- and B-cell immune responses.
The study is designed to evaluate the safety and immunogenicity of OVX033 at three dose levels. To date, no safety concerns or signals have been observed at any dose level, justifying a dose escalation up to the maximum dose level of 500µg.
The single-center, randomized, double-blind, placebo-controlled Phase 1 clinical study is being conducted at the Clinical Investigation Center in Vaccinology Cochin Pasteur in Cochin Hospital in Paris.
The French government supports this project through France 2030.
According to a recent study published by the journal Nature, coronaviruses (CoVs) are a group of enveloped viruses belonging to the Coronaviridae and currently contain four known genera: Alpha, Beta, Gamma, and Delta-CoVs. Sarbecovirus, a subgenus within Beta-CoV, has resulted in the emergence of the highly pathogenic human viruses SARS-CoV and SARS-CoV-2.

The Florida Department of Health (FDH) in Hillsborough County (DOH-Hillsborough) today announced it is informing Tampa-area residents of a confirmed human case of locally-acquired dengue fever.
DOH-Hillsborough and Hillsborough County Mosquito Control stated on June 24, 2024, that they coordinate surveillance and prevention efforts by aerial spraying.
DOH-Hillsborough is the third Florida country to report mosquito-transmitted dengue cases in 2024.
FDH published its Arbovirus Surveillance update #24 on June 15, 2024, disclosing seven locally acquired dengue cases have been reported from Miami-Dade, Pasco.
In 2024, 222 travel-associated dengue cases have been reported in Florida, primarily from visitors from Cuba and Brazil.
Florida continues statewide surveillance for mosquito-borne illnesses, including West Nile virus infections, Eastern equine encephalitis, St. Louis encephalitis, malaria, chikungunya, and dengue.
While the Dengvaxia vaccine is U.S. FDA-approved, it has limited available in the United States.

Novavax, Inc. today announced that it has filed for a type II variation of existing Marketing Authorization with the European Medicines Agency (EMA) for its JN.1 COVID-19 vaccine (NVX-CoV2705) for individuals aged 12 and older.
The submission follows guidance from EMA and the World Health Organization to target the JN.1 lineage for the Fall 2024 season.
Novavax's non-mRNA JN.1 COVID-19 vaccine targets the "parent strain" of KP.2 and KP.3.2
NVX-CoV2705 is an updated version of Novavax's authorized COVID-19 vaccine (NVX-CoV2373).
"Novavax is working closely with European markets seeking to offer a protein-based alternative to mRNA this fall for COVID-19 vaccination," said John C. Jacobs, President and Chief Executive Officer, Novavax, in a press release on June 24, 2024.
"Our updated COVID-19 vaccine is active against current circulating (SARS-CoV-2) strains, including KP.2 and KP.3."
Nonclinical data have demonstrated that Novavax's JN.1 COVID-19 vaccine induces broad neutralization responses to JN.1 lineage viruses, including those containing the F456L and R346T mutations, and to "FLiRT" and "FLuQE" variants.
Novavax's vaccine also produces conserved polyfunctional, Th1-biased CD4+ T cell responses to a range of JN.1 lineage variants.
Novavax confirmed it intends to have its JN.1 COVID-19 vaccine in unit-dose vials available for immediate release in the European Union after approval.
Novavax has also filed with the U.S. FDA and is working with other regulatory authorities globally to authorize or approve its JN.1 COVID-19 vaccine.

Since 2004, the Chikungunya virus (CHIKV) has caused large-scale outbreaks worldwide and has recently been identified in over 110 countries.
According to news from Valneva SE, a second country has approved IXCHIQ®, the world's first licensed chikungunya vaccine, to address this unmet medical need.
Valneva announced on June 24, 2024, that Health Canada had approved the IXCHIQ vaccine to prevent this mosquito-transmitted disease for adults.
This new approval follows the U.S. Food and Drug Administration decision in November 2023. The European Medicines Agency recently recommended marketing authorization for IXCHIQ in Europe, and a formal decision is expected in the third quarter of 2024.
On June 26, 2024, the U.S. CDC's vaccine committee plans to discuss how this innovative vaccine can be offered in the United States.
Chikungunya's economic burden is expected to increase as the mosquito vectors transmitting CHIKV spread geographically. As such, the World Health Organization recently highlighted chikungunya outbreaks as a significant public health problem.