Search API
Since the start of 2024, the Democratic Republic of Congo (DRC) has reported over 20,000 mpox cases, with more than 1,000 deaths, primarily affecting children.
In June 2024, the U.S. CDC issued a Level 2 Alert reporting a mpox outbreak in 25 out of 26 DTC provinces, including urban areas.
According to media sources, authorities in the DRC have responded to this outbreak by approving the use of two new vaccines.
AfricaNews.com reported on June 28, 2024, that emergency use authorization had been issued for the Jynneos® vaccine, developed by Bavarian Nordic, and LC16, produced by KM Biologics.
LC16 is a 3rd generation, live attenuated vaccine containing live vaccinia virus (LC16m8 strain) used to prevent smallpox and mpox.
The DRC decision follows rigorous evaluation by relevant authorities and stakeholders involved in the authorization process.
JYNNEOS (MVA-BN®, IMVAMUNE®) is a two-dose vaccine based on a live, attenuated vaccinia virus, Modified Vaccinia Ankara, and has been offered in the United States since May 2022.

Merck announced today that the U.S. CDC’s Advisory Committee on Immunization Practices (ACIP) unanimously voted to recommend CAPVAXIVE™ (Pneumococcal 21-valent Conjugate Vaccine) as an option for adults 65 years of age and older for pneumococcal vaccination.
Additionally, shared clinical decision-making is recommended regarding using a supplemental dose of CAPVAXIVE for adults 65 and older who have completed their vaccine series with both PCV13 and PPSV23.
“CAPVAXIVE represents an innovative approach to invasive pneumococcal disease prevention in adults, as it is specifically designed to help protect against the strains that cause the majority of severe disease in adults 65 years of age and older,” said Dr. Eliav Barr, senior vice president, Merck Research Laboratories, in a press release on June 27, 2024.
“The ACIP vote recognizes the clinical profile of CAPVAXIVE for adults in the U.S., and we look forward to the CDC’s final, published recommendations.”

PharmaJet® today announced that their Tropis® Intradermal (ID) Needle-free System will be used in a house-to-house polio immunization campaign.
Over a quarter million PharmaJet’s needle-free intradermal syringes have been provided to support this initiative.
The campaign will be conducted in two rounds to reduce the immunity gap significantly against type-2 poliovirus. Young children will receive the needle-free polio vaccine and novel oral polio vaccine (nOPV2) to achieve 95% coverage in each round.
The polio campaign, a collaboration of the African Field Epidemiology Network, WHO, UNICEF, BMGF, GAVI, and U.S. CDC, targets over 170,000 children in Somalia.
The most recent evidence for human circulating vaccine-derived polio virus-2 was in March 2024.
Through the Somalia Emergency Action Plan, the country will continue to work with humanitarian partners to reach about 1.5 million zero-dose children, most of whom live in the country’s highly populated central and southern areas.
Paul LaBarre, Vice President of Global Business Development at PharmaJet, commented in a press release on June 27, 2024, “In Somalia, we are eager to build on previous house-to-house campaign experience that demonstrates how needle-free enables vaccination teams to move quickly and achieve high coverage without the burden of sharps waste management and with reduced vaccine volume and cold chain logistics.”
The U.S. CDC reissued a Global Polio Alert on May 23, 2024, regarding polio outbreaks and poliovirus detections in 34 countries. The CDC recommends that visitors to these countries be fully vaccinated against polio.

As the three-day Advisory Committee on Immunization Practices (ACIP) meeting ended today, the morning session focused on Respiratory syncytial virus (RSV), the leading cause of hospitalization among U.S. infants.
Led by Sarah S. Long, MD, the Maternal/Pediatric RSV Work Group presentations included the summary of the effectiveness of Beyfortus™ (nirsevimab) in infants.
Beyfortus is a single-dose, extended half-life monoclonal antibody (mAb) that offers passive immunization to prevent lower respiratory tract infections. It has been approved by the U.S. FDA and other health agencies.
On June 28, 2024, Amanda Payne, PhD, MPH, stated that Beyfortus was about 80% effective against RSV-associated encounters and hospitalizations among infants in their first RSV season during the 2023-2024 RSV season.
Furthermore, the U.S. CDC's RSVVaxView recently reported that among females with a young infant, over 43% reported that their infant received Beyfortus.
The ACIP group, which comprises vaccine experts, loudly expressed its enthusiasm for the effectiveness and uptake of this first-year mAb therapy.
The group's primary concern was product availability for the 2024-2025 RSV season.
While Beyfortus was available in the U.S. for the 2023-2024 RSV season, demand quickly outstripped supply.
Beyfortus's producers, Sanofi and AstraZeneca, confirmed on May 2, 2024, that the expansion of the manufacturing network is progressing. In late 2024, the companies could have more than tripled their manufacturing capacity and increased mAb supply.
Of note, should Beyfortus production fall behind demand during the next RSV season, the U.S. FDA has approved a vaccine that pregnant women can receive, which enables antibodies to be passed to the unborn child.

Dynavax Technologies Corporation today announced that the first participant has been dosed in a Phase 1/2 clinical trial evaluating the safety, tolerability, and immunogenicity of Z-1018, the company's investigational vaccine candidate being developed for the prevention of shingles (herpes zoster).
The Phase 1/2 randomized, active-controlled, dose escalation, multicenter trial is expected to enroll approximately 440 healthy adults aged 50 to 69 years at trial sites in Australia and will evaluate the safety, tolerability, and immunogenicity of Z-1018 compared to the Shingrix® vaccine.
Key objectives of the trial include selecting the optimal glycoprotein E (gE) protein dose level and dosing schedule for further clinical development. The Phase 1/2 trial will also support the validation of a Patient-Reported Outcome measurement tool to differentiate Z-1018 on tolerability and support potential label claims.
"We believe there is an opportunity to develop an improved shingles vaccine with a significantly better tolerability profile than the market-leading shingles vaccine. One of the unique advantages of our vaccine candidate is CpG 1018 adjuvant's established safety and tolerability profile, combined with its ability to induce strong CD4+ T-cell responses, which are thought to be critical in preventing the reactivation of the herpes zoster virus," said Rob Janssen, M.D., Chief Medical Officer of Dynavax, in a press release on June 27, 2024.
Dynavax anticipates reporting top-line immunogenicity and safety data in the second half of 2025, including comparing CD4+ T-cells one month after the second of two vaccine doses.
According to the U.S. CDC, shingles risk increases with age and in people with weakened immune systems. About 33% of people in the United States develop shingles at least once, and fewer than 100 people die of shingles each year.
As of June 2024, there are four approved shingles vaccines and several vaccine candidates conducting clinical research.

With a third respiratory syncytial virus (RSV) vaccine approved by the U.S. FDA, many people ask which one offers the best protection from disease. With the 2024-2025 RSV season fast approaching, the U.S. CDC's recent vaccine meeting helped answer questions.
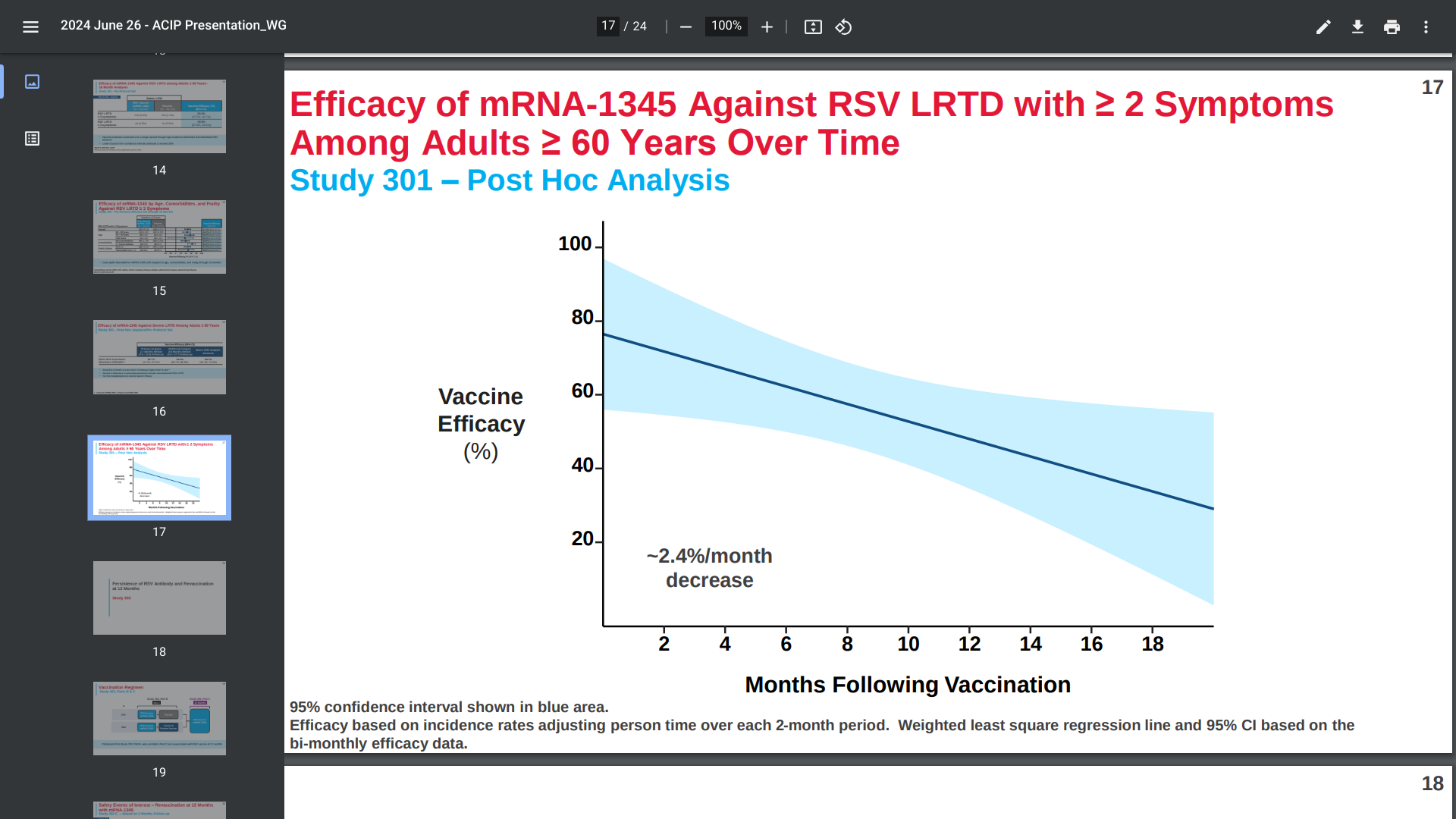
Moderna's presentation on June 26, 2024, to the CDC’s Advisory Committee on Immunization Practices, led by Rituparna Das, MD, PhD, disclosed that mRESVIA® (mRNA-1345) showed about 81% efficacy after 3.7 months, but around 50% (37.5%, 60.7%) efficacy in preventing illness after 18 months.
Additionally, Moderna stated that the RSV vaccine is generally well tolerated in over 19,700 adults over 60 years old vaccinated with a 50g licensed dose and that there are no safety concerns.
mRESVIA is an RSV vaccine containing an mRNA sequence encoding a stabilized prefusion F glycoprotein. It uses the same lipid nanoparticles as Moderna's other approved vaccine.
As of May 2024, an estimated 24.4% (95% Confidence Interval: 23.7%-25.2%) of adults 60 years and older reported receiving an RSV vaccine during the last RSV season.
During Walmart Health Center's five-year journey, people saved money and had better access to healthcare providers and enhanced services such as travel vaccinations.
Effective July 1, 2024, Walmart is closing all 51 health centers across five states and its virtual care offering.
Despite the closure of its health centers and virtual care service, Walmart continues to operate nearly 4,600 pharmacies, which offer various screenings and vaccines during the summer of 2024.
According to the NCPA, independent pharmacies remain a top vaccination destination in the U.S.

GSK plc today announced that the US Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) voted in favour of recommending the routine use of Respiratory Syncytial Virus (RSV) vaccines in all adults aged 75 and above.
The ACIP also recommended RSV immunization for adults aged 60-74 who are at increased risk for severe RSV disease.
These recommendations replace the previous recommendation for shared clinical decision-making in these age groups and have the potential to positively impact access to RSV immunization, particularly for the estimated 23 million U.S. adults aged 75 and older.
In May 2023, the U.S. FDA approved GSK’s AREXVY™ RSV vaccine, which is currently available at clinics and pharmacies before the 2024-2025 RSV season.
The ACIP recommendations will be forwarded to the director of the CDC and the Department of Health and Human Services for review and approval. Once approved, the final recommendations will be published in a future CDC Morbidity and Mortality Weekly Report.

While the first day of the Advisory Committee on Immunization Practices (ACIP) meeting was focused on Respiratory Syncytial Virus vaccines, day #2's agenda focuses on respiratory diseases.
On June 27, 2024, the U.S. CDC's vaccine committee meeting agenda includes presentations on updated COVID-19, influenza vaccine options, and a new pneumococcal vaccine (PCV21).
With the 2024-2025 respiratory season fast approaching in the Northern Hemisphere, the general public can listen to today's clinical discussions and votes at this YouTube link.
The ACIP develops recommendations for U.S. immunizations, including ages when vaccines should be given, number of doses, time between doses, and precautions and contraindications.
If the recommendations are adopted by the CDC Director Mandy K. Cohen, MD, MPH, they will be published in a CDC MMWR.

