Search API
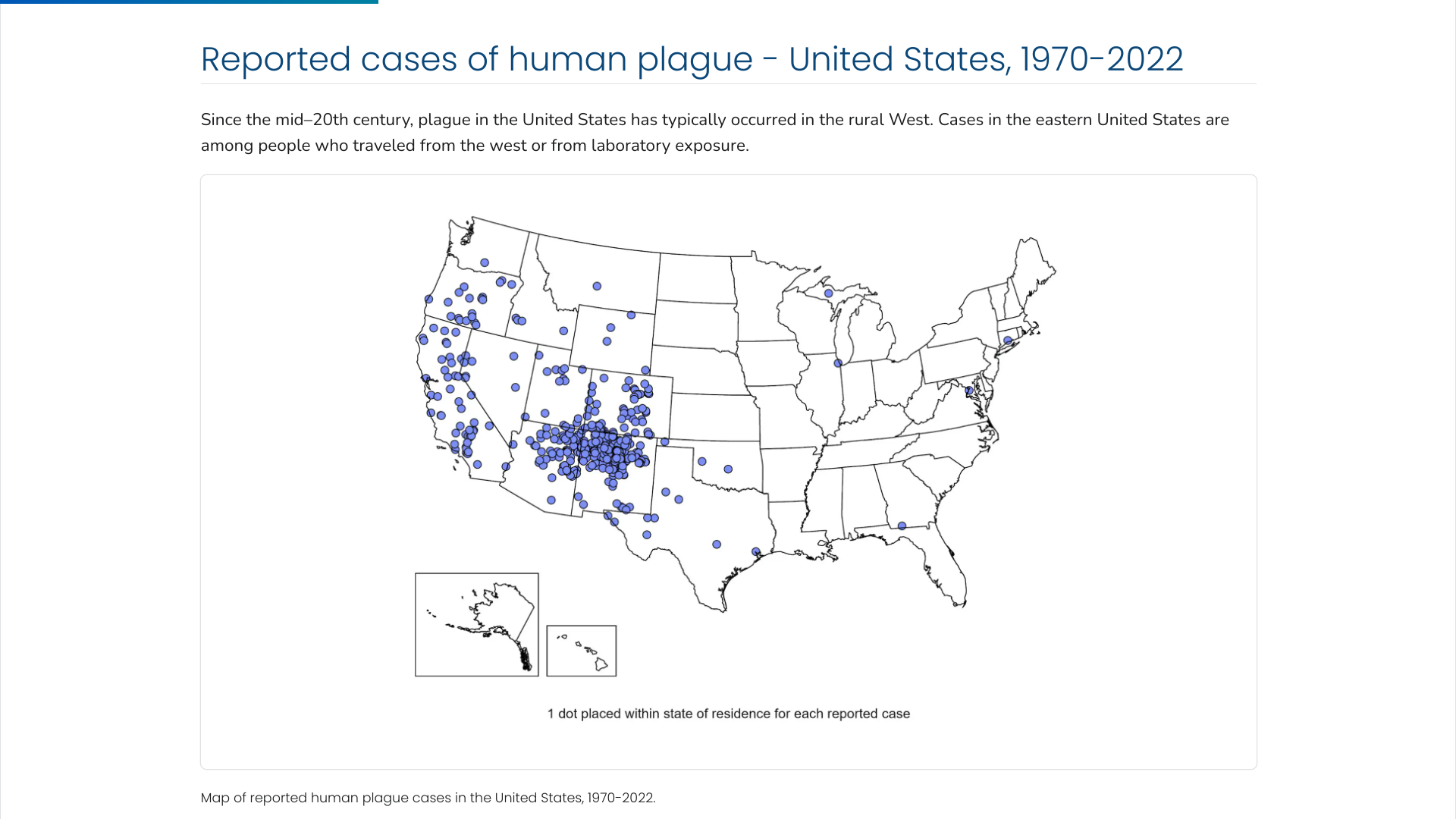
The U.S. Centers for Disease Control and Prevention (CDC) says the Plague was first introduced into the United States in 1900. The plague bacterium (Yersinia pestis) is transmitted by fleas and cycles naturally among wild rodents.
Over the decades, the Plague spread from urban rats to rural rodent species and became entrenched in many areas of the western U.S.
Almost all of the cases reported in the last 20 years have occurred among people living in small towns and villages or agricultural areas rather than in larger towns and cities, says the CDC.
As of 2024, the CDC estimates that seven human cases of Plague occur in the U.S. each year.
Recent plague cases include the Pueblo Department of Public Health and Environment confirming a human case of Plague in a Pueblo County resident on July 9, 2024.
And in February 2024, health officials in Oregon reported a case of bubonic Plague in a resident who they said likely contracted it from a pet cat.
Globally, the most human plague cases since the 1990s have occurred in Africa.
From a prevention perspective, plague vaccines are no longer available in the U.S. However, plague vaccine candidates are in development but are not expected to be commercially available in the immediate future.
In March 2023, the first mRNA-based, lipid nanoparticle vaccine was found effective against lethal bacteria in mice.
As numerous vaccine manufacturers strive to produce improved flu shot options, innovative influenza vaccine candidates post positive results in 2024.
Intranasal vaccines are known to provide a wall of defense at the site of infection, helping prevent influenza viruses from entering the nasal mucosa.
FluGen, Inc., today announced the results of its study comparing the coadministration of intranasal M2SR and the high-dose flu shot in older adults ages 65-85.
Published in the journal Lancet Infectious Diseases on July 11, 2024, the study, funded by the U.S. Department of Defense, evaluated the safety, tolerability, and immunological response to FluGen’s investigational supra-seasonal, live, single-replication, intranasal influenza vaccine when administered with Fluzone High Dose inactivated influenza vaccine.
These researchers concluded the H3N2 M2SR vaccine coadministered with Fluzone HD in older adults was well tolerated and provided enhanced immunogenicity compared with Fluzone HD administered alone.
This finding suggests the potential for improved influenza vaccination efficacy in this age group.
“The idea of delivering two vaccines in one sitting has become widely accepted,” said Paul Radspinner, President and Chief Executive Officer of FluGen, in a press release on July 12, 2024.
“Imagine being at your local pharmacy for your annual flu shot and also receiving a quick nasal spray that would greatly enhance your chances not only of becoming seriously ill but of being infected at all. This combination solution could have a tremendous impact on the health of older adults."
Radspinner went on to discuss the possible impact on influenza pandemic protection. “If H5N1 or any other mutating influenza strain were to begin infecting millions of people, imagine the benefits of combining an intranasal vaccine, which could stop most infections from occurring, with a strong antibody-based vaccine shot."
"The impact on human health could be unequaled in our history.”
As of July 2024, new trivalent influenza vaccines, such as Flucelvax, had been shipped to pharmacies before the 2024-2025 flu season launches in the United States.

Tiba Biotech LLC today announced its new partnership with the Biomedical Advanced Research and Development Authority (BARDA) through the initiation of an EZ-BAA contract to develop groundbreaking therapeutics against influenza.
The U.S. $749,999 BARDA contract supports early-stage therapeutic platform development for the Flexible and Strategic Therapeutics program.
This BARDA initiative aims to advance cutting-edge therapeutic platform technologies, such as RNA-mediated interference, which could be rapidly deployed in response to emerging viral threats. The therapeutic will target the highly conserved viral nucleoprotein and will be delivered via Tiba’s RNABLTM platform.
Tiba Biotech’s initial focus will be developing a prototype RNAi-based therapeutic for H1N1 influenza (swine flu), a type of influenza A virus.
Every year, there are rare, sporadic human infections with influenza viruses that usually circulate in pigs and not people, says the U.S. CDC.
On June 28, 2024, the CDC reported the second and third U.S. human infections in 2024.
This initiative is a natural extension of Tiba’s ongoing work combating influenza pandemic threats, most notably in the form of a novel multi-antigen mRNA-based H7N9 flu vaccine funded under a Phase II SBIR award from the National Institutes of Health and ongoing collaborations with the Collaborative Influenza Vaccine Innovation Centers.
Tiba was also recently accepted into BLUE KNIGHT™, a joint initiative between Johnson & Johnson Innovation—JLABS and BARDA. The initiative aims to harness innovation to combat future known and unknown health threats.

New data published today by the UK Health Security Agency (UKHSA) shows whooping cough cases continue to increase in England, reaching 7,599 at the end of May 2024.
On July 11, 2024, the UKHSA also confirmed there have been nine infant deaths since the current whooping cough (pertussis) outbreak began in November last year.
Whooping cough is a cyclical disease that peaks every 3 to 5 years. The last cyclical increase in England occurred in 2016.
The UKHSA says timely vaccination in pregnancy and childhood is important to protect vulnerable young infants from serious disease.
Dr. Mary Ramsay, Director of Immunisation at the UKHSA, said in a press release, "Vaccination is the best defense against whooping cough, and it is vital that pregnant women and young infants receive their vaccines at the right time."
The latest uptake data for the vaccination offered to pregnant women to protect newborn infants against whooping cough continues to decline, with coverage at 58.9% in March 2024 compared to the peak coverage (72.6%) in March 2017.
Evidence from England shows that vaccination at the right time in pregnancy is highly effective, giving 92% protection against infant death.
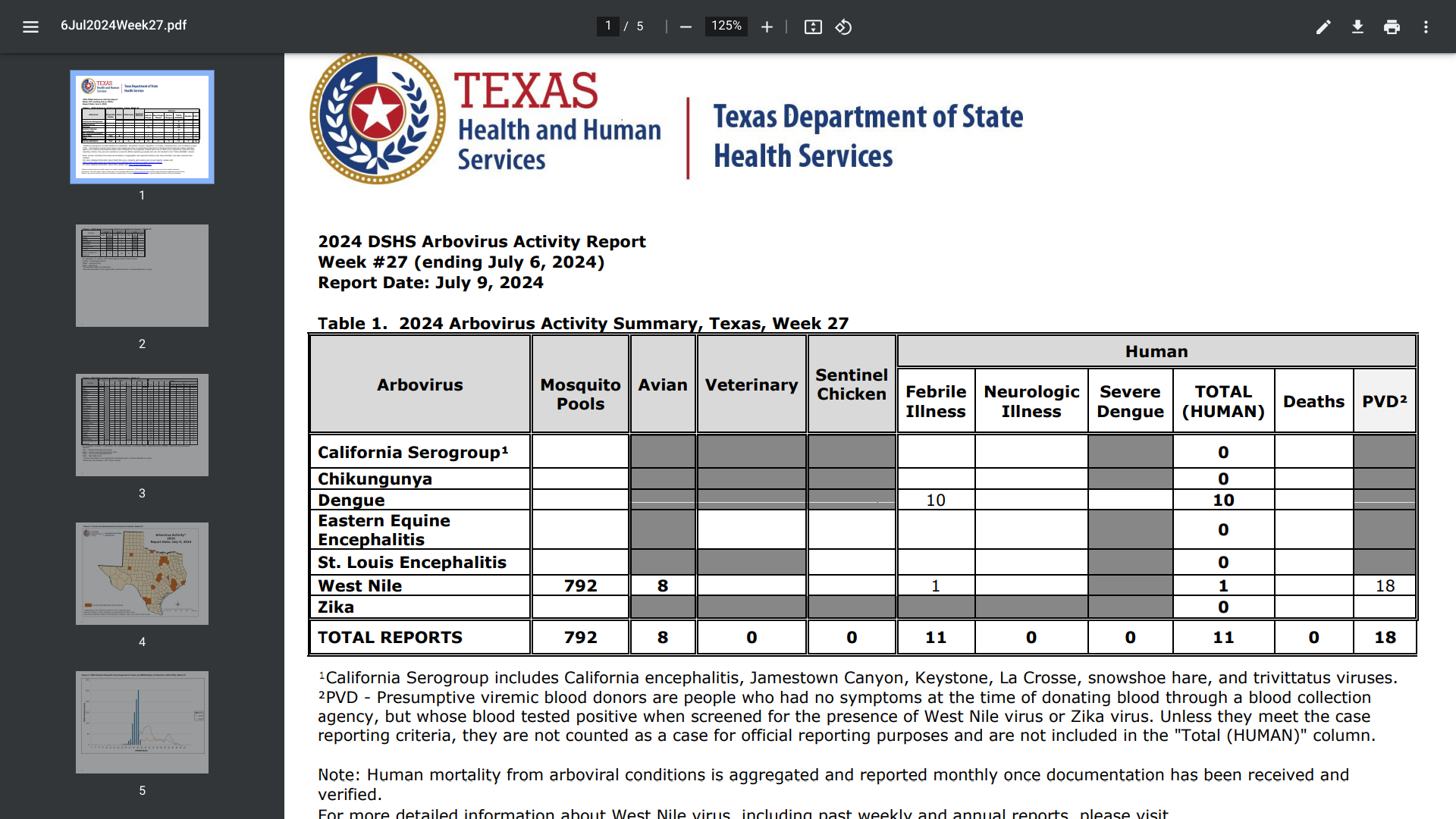
Texas public health officials announced today that they are urging Texans to protect against mosquito bites after confirming ten travel-associated dengue cases for 2024.
As of July 11, 2024, all of the dengue cases reported in Texas this year were acquired while traveling internationally.
However, a small number of dengue cases have been acquired from mosquito bites in southern Texas in recent years. Mosquitoes that carry the dengue virus are found in both Mexico and Texas.
The Texas Department of State Health Services (DSHS) confirmed the new dengue cases were reported in Collin, Dallas, Fort Bend, McMullen, Montgomery, and Travis counties.
In 2023, there were 79 cases of dengue in Texas, including one locally acquired case in Val Verde County.
To the south of Texas, Mexico has reported about 1,000 dengue cases in 2024. In 2023, Mexico reported over 277,000 dengue cases.
“Unfortunately, many mosquitoes in Texas can spread diseases, such as West Nile and dengue. These diseases are often mild, but some people will develop severe illness,” said DSHS Commissioner Jennifer Shuford, MD, MPH, in a press release.
According to DSHS, about 25% of dengue infections become symptomatic.
Most people recover completely within two weeks. However, about one in 20 symptomatic people develop a severe infection called Dengue Hemorrhagic Fever that can be fatal if untreated.
From a prevention perspective, two dengue vaccines are used in various countries but not in the U.S. as of July 11, 2024.
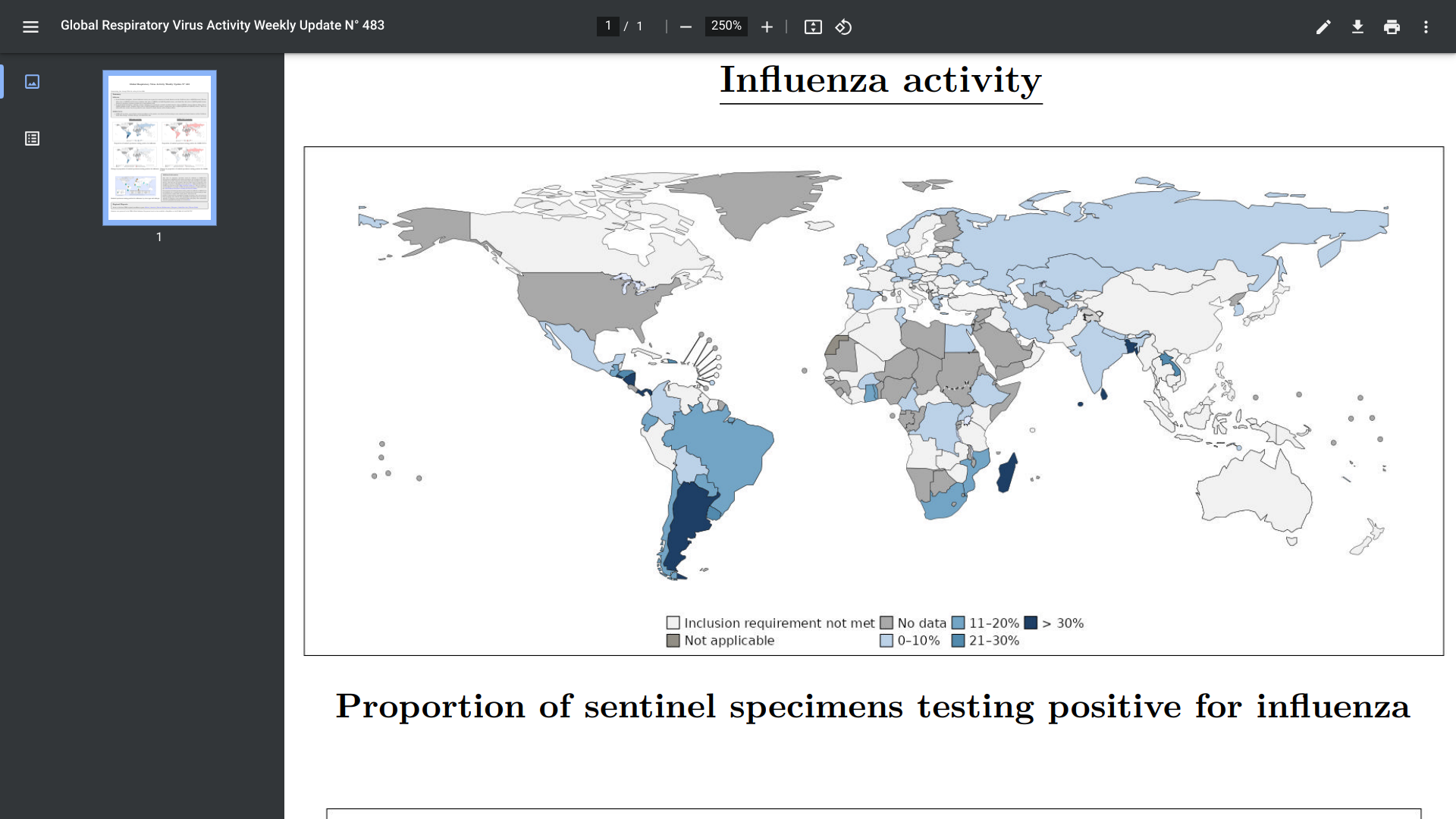
According to the World Health Organization (WHO) Influenza Update N° 483, elevated influenza activity was reported in countries in Central America and the Caribbean, Western Africa, Southern Asia, and South East Asia due to various virus types.
As of July 10, 2024, in the Southern Hemisphere, influenza activity continues to be elevated in South America and Oceania countries. There are indications that activity may have peaked in some South American and Southern African countries.
In the United States, influenza case reports for the 2024-2025 flu season are reduced.
However, flu shots for the new season have already been distributed to pharmacies in the U.S.
Biotechnology company Apriori Bio recently announced it was awarded $1.1 million from the Coalition for Epidemic Preparedness Innovations (CEPI) to advance its biology-informed artificial intelligence platform Octavia™, aimed at protecting humanity against rapidly evolving viruses by designing variant-resilient vaccines.
The new seed funding from CEPI will focus on pandemic influenza strain H3N2, which has previously affected pigs, birds, and humans.
The Octavia platform works by characterizing large libraries of viral variants on their ability to bind to cells in the human body and evade the immune response. Then, using machine learning, Octavia generates maps to identify the mutations that have the greatest ‘escape potential’ and could, therefore, pose the greatest threat.
Octavia builds and trains its algorithms using computational insights and experimental biological data. This includes studying viral evolutionary trees to identify the point at which viral variability is most likely to occur and exploring how mutations could affect each other.
These insights can guide the design and updating of new vaccines and existing vaccines so that they can protect against worrisome variants for years to come.
Lovisa Afzelius, Ph.D., MBA, Co-founder and CEO of Apriori, said in a press release on July 7, 2024, “We are honored to be recognized by a global leader in pandemic preparedness who shares our commitment to better protecting humanity from rapidly evolving viruses."
CEPI’s 2022-2026 plan, known as CEPI 2.0, will help the world make the scientific progress needed to respond to the next Disease X threat with a new vaccine in just 100 days. This goal is known as the 100 Days Mission.



