Search API
Today marks the official rollout of the newly approved R21/Matrix-M™ malaria vaccine, co-developed by the University of Oxford and Serum Institute of India (SII), which has committed to producing 100 million vaccines.
This malaria vaccine utilizes Novavax’s Matrix-M™ adjuvant technology.
The first official vaccination is scheduled for July 15, 2024, in Abidjan, Côte d’Ivoire, and it will be subsequently introduced in 38 districts across the country.
According to a press release, 15 African countries will introduce malaria vaccines with Gavi support in 2024. These countries plan to reach around 6.6 million children with the malaria vaccine in 2024 and 2025.
John Jacobs, President and Chief Executive Officer of Novavax Inc., commented, "The introduction of the R21/Matrix-M™ malaria vaccine in Côte d'Ivoire marks a breakthrough in the fight to protect vulnerable children against a leading cause of death across the region while reinforcing our mission to create innovative vaccines that improve public health."
"Novavax is proud of the contribution of our Matrix-M™ adjuvant in this vaccine and in making this moment possible, and value our continued collaboration with the University of Oxford and SII, as well as the lifesaving work of WHO, Gavi, and UNICEF.”
R21/Matrix-M is a low-dose, highly effective, and affordable vaccine that can be manufactured quickly and scale. Ghana, Nigeria, Burkina Faso, and the Central African Republic have approved the new vaccine, and many others are preparing to receive shipments.
Malaria vaccines are currently unavailable in the United States.
Novavax, based in Gaithersburg, MD., U.S., promotes improved health by discovering, developing, and commercializing innovative vaccines to help protect against serious infectious diseases.
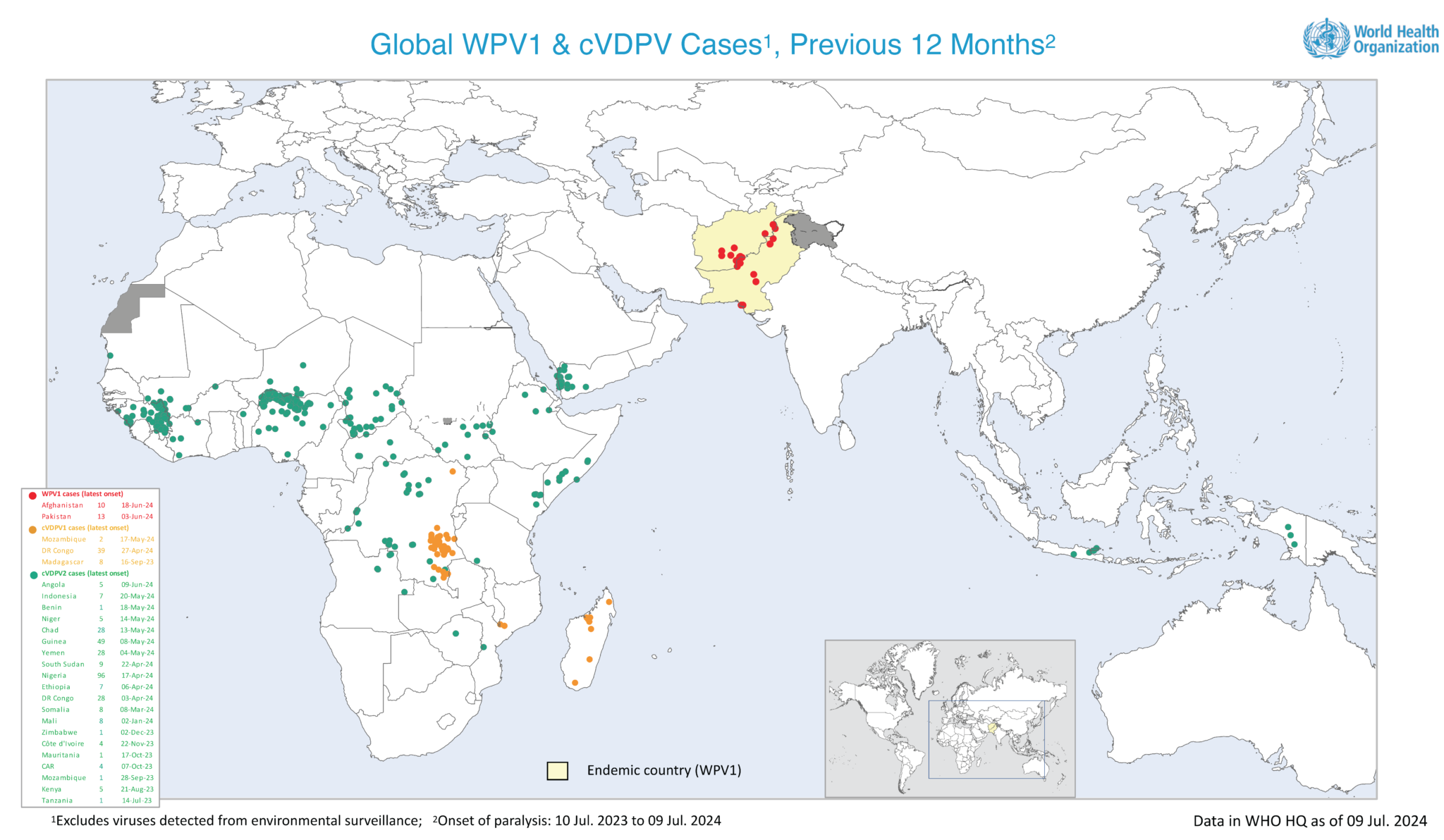
According to the Global Polio Eradication Initiative (GPEI), a joint Polio Independent Monitoring Board and Transition Independent Monitoring Board meeting will be held in Geneva, Switzerland, beginning July 15, 2024.
The goal of the meeting is to assess the challenges of eliminating polio in the endemic and outbreak countries.
Unfortunately, the GPEI reported three additional wild poliovirus type 1 (WPV1) cases in Afghanistan last week, bringing the country's total for 2024 to nine.
In 2023, Afghanistan reported six WPV1 cases.
In this region, Pakistan, which is also fighting the speed of poliovirus, reported eight new WPV1 environmental detections last week.
In addition to these polio disclosures, the U.S. CDC says to interrupt poliovirus transmission, a renewed focus on increasing routine immunization coverage in endemic areas and implementing higher-quality supplementary immunization activities is necessary.
Over the last decade, about 10 billion doses of oral polio vaccine were administered worldwide, reports the GPEI.
The CDC's Global Polio Travel Advisory (May 2024) recommends that before visiting any of the 34 destinations, adults who completed the full, routine polio vaccine series may receive a single, life booster dose of polio vaccine.
In the United States, polio vaccines are generally available at pharmacies and travel clinics.
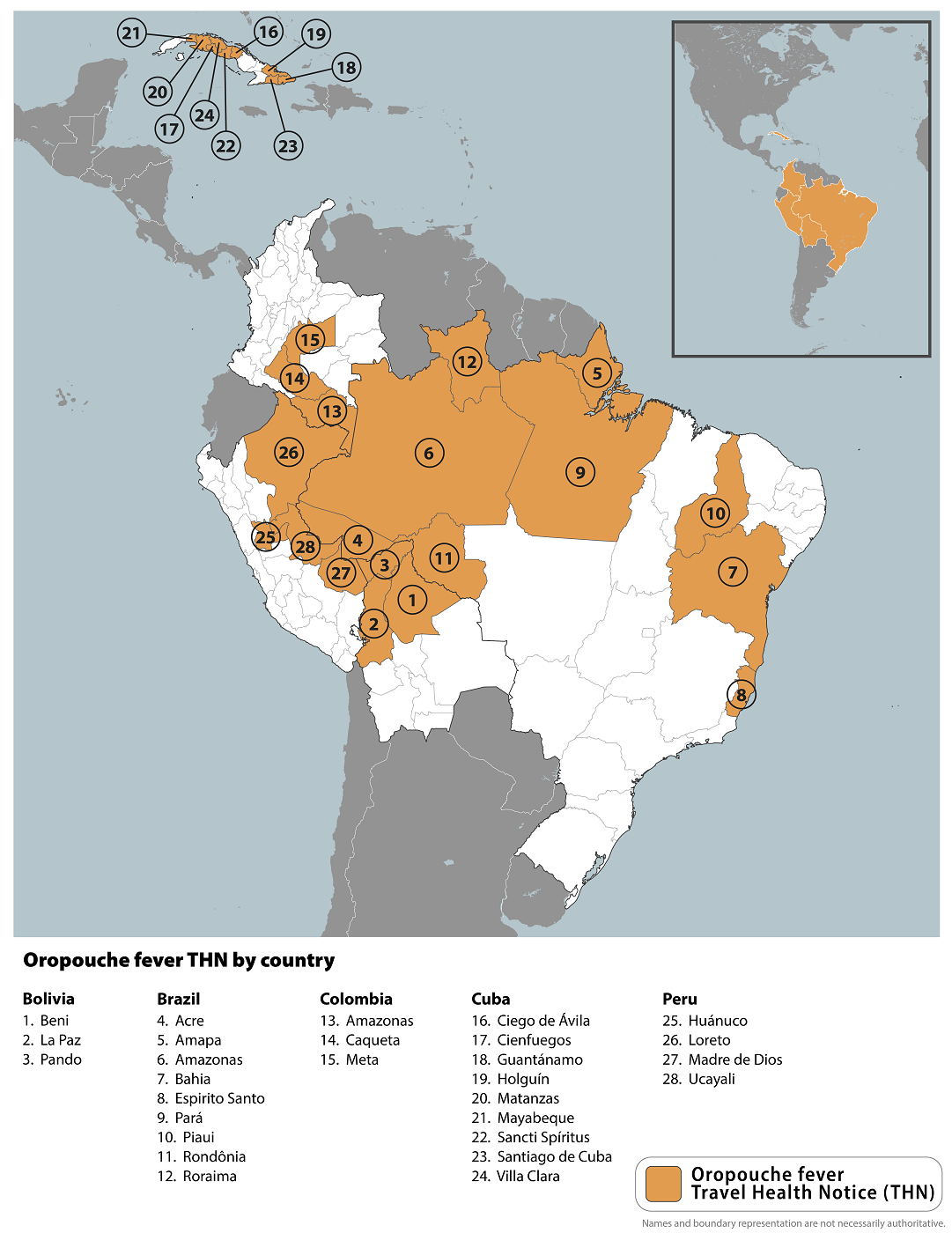
The U.S. CDC reissued a global warning about Oropouche fever outbreaks in various communities of Brazil, Bolivia, Colombia, Peru, and Cuba in the Region of the Americas.
In late June 2024, the CDC said travelers should seek medical care if they develop high fever, headache, muscle aches, stiff joints, nausea, vomiting, chills, or sensitivity to light during or after travel.
For example, the Ministry of Public Health of Cuba reported the first-ever outbreak of Oropouche virus disease in May, confirming 74 cases from the Province of Santiago de Cuba (54) and the Province of Cienfuegos (20).
Oropouche virus is primarily transmitted to humans through the bite of the midge, Culicoides paraensis, but the mosquito Culex quinquefasciatus can also transmit it.
The Pan-American Health Organization / World Health Organization urges Member States to intensify surveillance given its clinical presentation and considering the current situation of chikungunya, dengue, zika, and other common vector-borne diseases in the Region.
While there are approved vaccines to prevent chikungunya and dengue diseases, Oropouche and zika viruses do not have vaccines available in July 2024.
The board of directors of CSPC Pharmaceutical Group Limited announced on July 11, 2024, that the mRNA Respiratory Syncytial Virus (RSV) vaccine candidate (SYS6016) has obtained approval from the National Medical Products Administration of the People’s Republic of China to conduct human clinical trials in China.
Currently, there is no vaccine available in China that protects people from RSV infection.
In preclinical studies, SYS6016 translated into the prefusion conformation F-protein in vivo and induced high titers of long-lasting neutralizing antibodies.
CSPC wrote that this vaccine candidate exhibits good protection against RSV-A and RSV-B subtype viral strains and has a good safety profile.
CSPC confirmed it would endeavor to advance the clinical research and market SYS6016 as soon as possible to create value for society and shareholders.
As of July 13, 2024, three RSV vaccines and one monoclonal antibody for infants (Beyfortus) were approved for use in the United States.

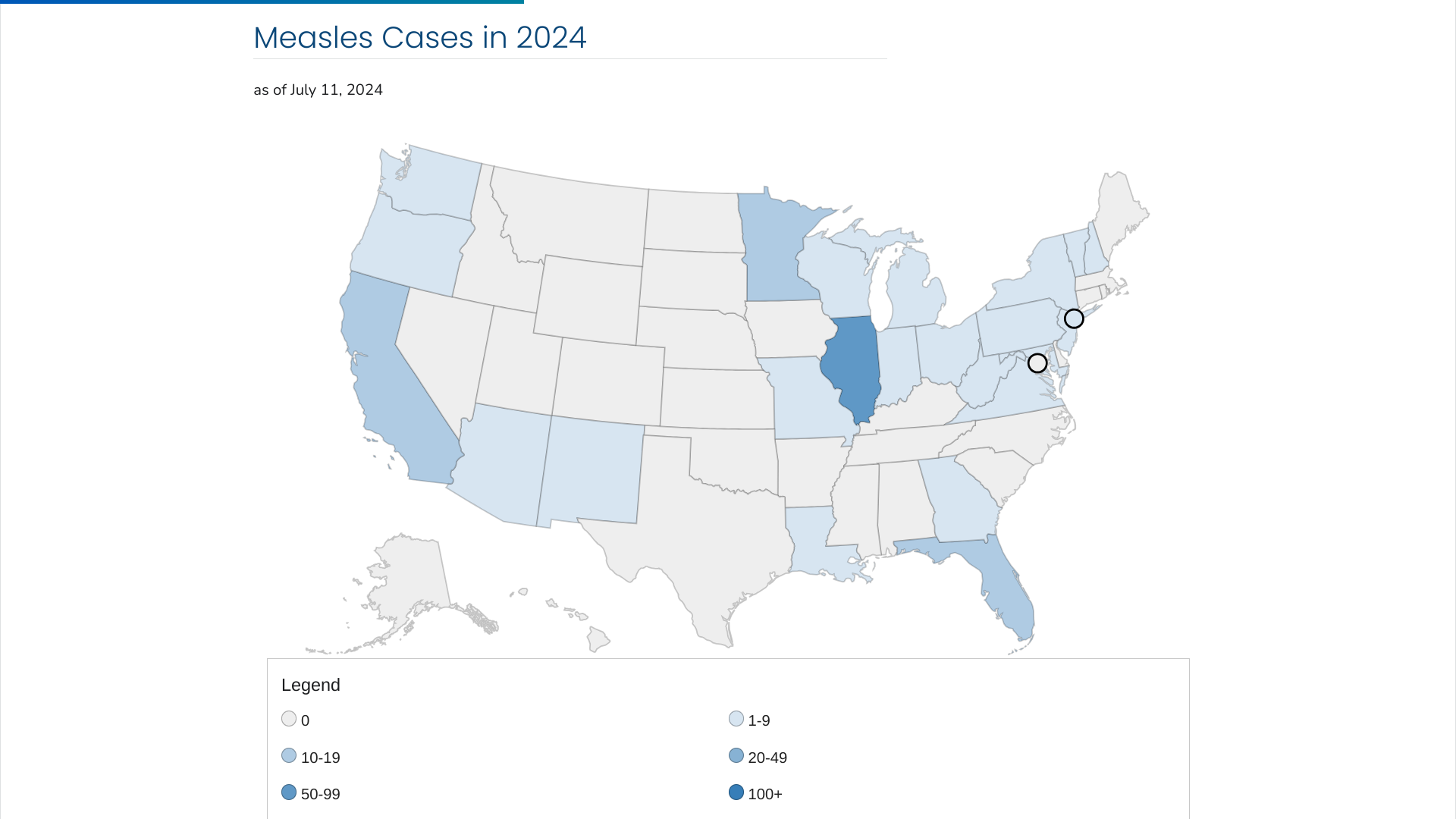
The Michigan Department of Health and Human Services (MDHHS) and the Macomb County Health Department today reported the sixth case of measles in the state in 2024.
The most recent case reported north of Detroit on July 12, 2024, had no known international travel association.
The U.S. Centers for Disease Control and Prevention (CDC) recently warned about possible travel-related measles outbreaks amid a global rise in cases.
On July 11, 2024, the CDC confirmed 167 cases have been reported this year, far outpacing the 58 measles cases reported in 2023.
In 2024, 84% of measles cases were in unvaccinated people or had an unknown vaccination status.
Throughout Michigan, pharmacies and clinics are offering various measles-preventing vaccines.
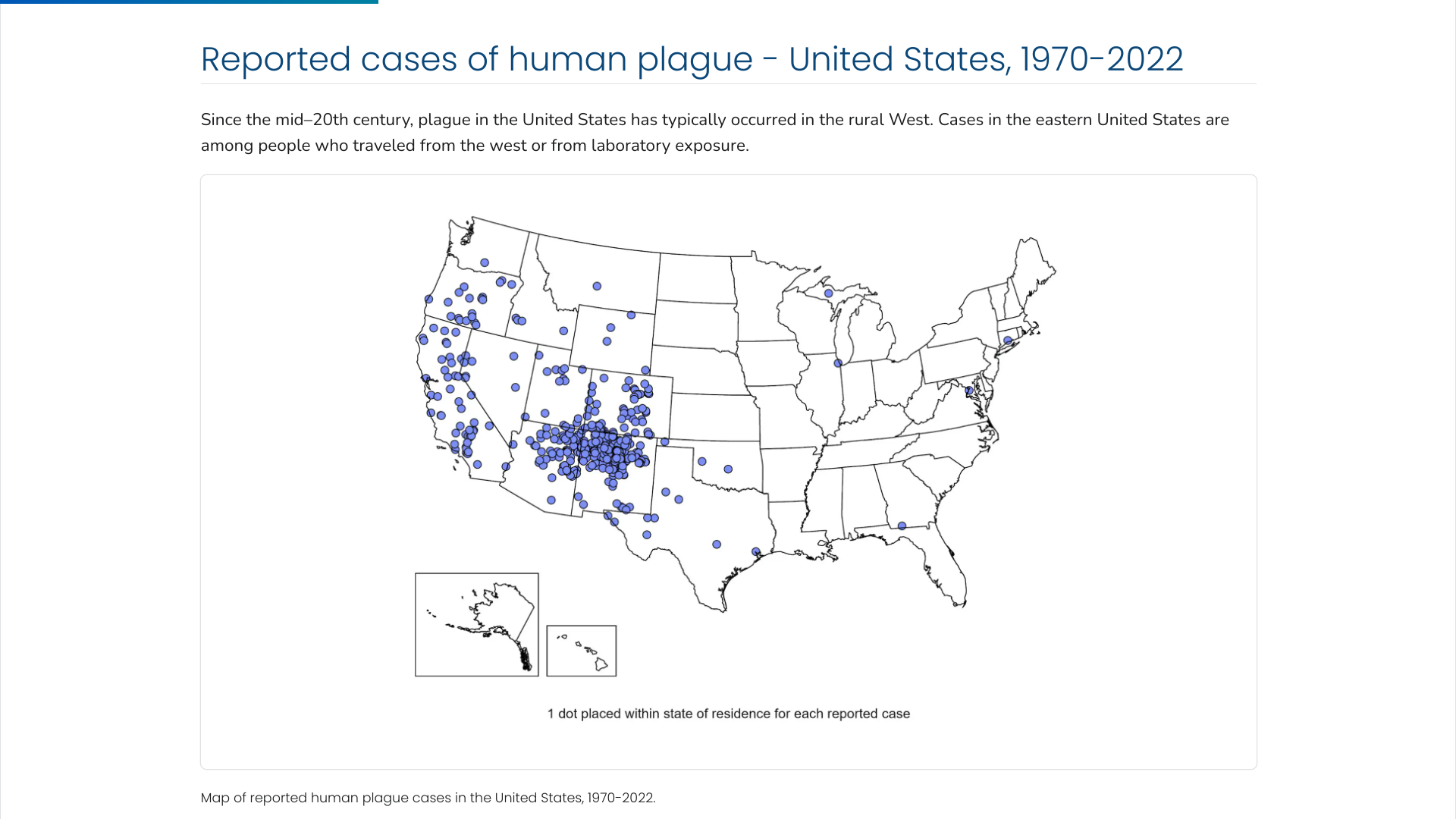
The U.S. Centers for Disease Control and Prevention (CDC) says the Plague was first introduced into the United States in 1900. The plague bacterium (Yersinia pestis) is transmitted by fleas and cycles naturally among wild rodents.
Over the decades, the Plague spread from urban rats to rural rodent species and became entrenched in many areas of the western U.S.
Almost all of the cases reported in the last 20 years have occurred among people living in small towns and villages or agricultural areas rather than in larger towns and cities, says the CDC.
As of 2024, the CDC estimates that seven human cases of Plague occur in the U.S. each year.
Recent plague cases include the Pueblo Department of Public Health and Environment confirming a human case of Plague in a Pueblo County resident on July 9, 2024.
And in February 2024, health officials in Oregon reported a case of bubonic Plague in a resident who they said likely contracted it from a pet cat.
Globally, the most human plague cases since the 1990s have occurred in Africa.
From a prevention perspective, plague vaccines are no longer available in the U.S. However, plague vaccine candidates are in development but are not expected to be commercially available in the immediate future.
In March 2023, the first mRNA-based, lipid nanoparticle vaccine was found effective against lethal bacteria in mice.