Search API
According to the Global Polio Eradication Initiative (GPEI), a joint Polio Independent Monitoring Board and Transition Independent Monitoring Board meeting will be held in Geneva, Switzerland, beginning July 15, 2024.
The goal of the meeting is to assess the challenges of eliminating polio in the endemic and outbreak countries.
Unfortunately, the GPEI reported three additional wild poliovirus type 1 (WPV1) cases in Afghanistan last week, bringing the country's total for 2024 to nine.
In 2023, Afghanistan reported six WPV1 cases.
In this region, Pakistan, which is also fighting the speed of poliovirus, reported eight new WPV1 environmental detections last week.
In addition to these polio disclosures, the U.S. CDC says to interrupt poliovirus transmission, a renewed focus on increasing routine immunization coverage in endemic areas and implementing higher-quality supplementary immunization activities is necessary.
Over the last decade, about 10 billion doses of oral polio vaccine were administered worldwide, reports the GPEI.
The CDC's Global Polio Travel Advisory (May 2024) recommends that before visiting any of the 34 destinations, adults who completed the full, routine polio vaccine series may receive a single, life booster dose of polio vaccine.
In the United States, polio vaccines are generally available at pharmacies and travel clinics.
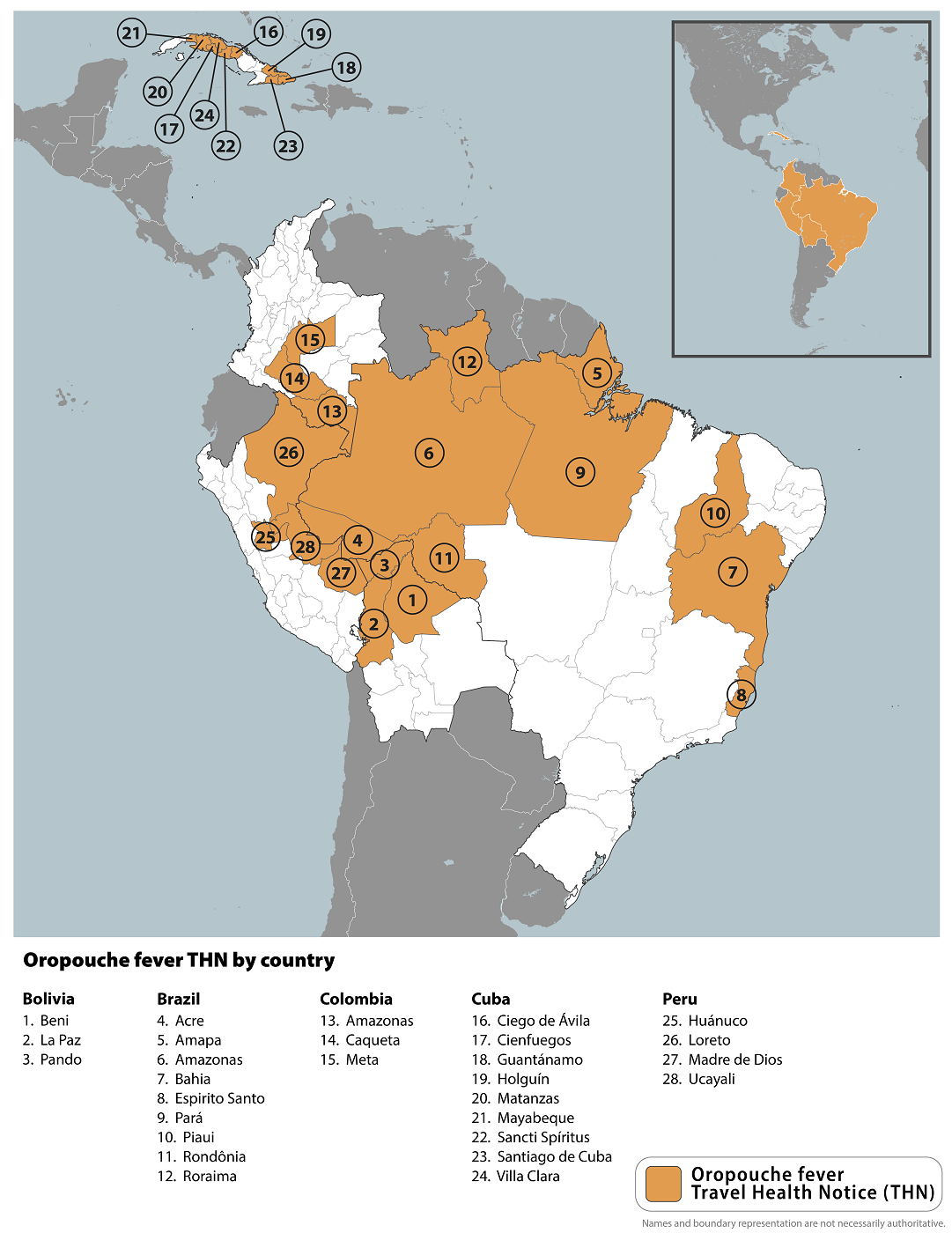
The U.S. CDC reissued a global warning about Oropouche fever outbreaks in various communities of Brazil, Bolivia, Colombia, Peru, and Cuba in the Region of the Americas.
In late June 2024, the CDC said travelers should seek medical care if they develop high fever, headache, muscle aches, stiff joints, nausea, vomiting, chills, or sensitivity to light during or after travel.
For example, the Ministry of Public Health of Cuba reported the first-ever outbreak of Oropouche virus disease in May, confirming 74 cases from the Province of Santiago de Cuba (54) and the Province of Cienfuegos (20).
Oropouche virus is primarily transmitted to humans through the bite of the midge, Culicoides paraensis, but the mosquito Culex quinquefasciatus can also transmit it.
The Pan-American Health Organization / World Health Organization urges Member States to intensify surveillance given its clinical presentation and considering the current situation of chikungunya, dengue, zika, and other common vector-borne diseases in the Region.
While there are approved vaccines to prevent chikungunya and dengue diseases, Oropouche and zika viruses do not have vaccines available in July 2024.
The board of directors of CSPC Pharmaceutical Group Limited announced on July 11, 2024, that the mRNA Respiratory Syncytial Virus (RSV) vaccine candidate (SYS6016) has obtained approval from the National Medical Products Administration of the People’s Republic of China to conduct human clinical trials in China.
Currently, there is no vaccine available in China that protects people from RSV infection.
In preclinical studies, SYS6016 translated into the prefusion conformation F-protein in vivo and induced high titers of long-lasting neutralizing antibodies.
CSPC wrote that this vaccine candidate exhibits good protection against RSV-A and RSV-B subtype viral strains and has a good safety profile.
CSPC confirmed it would endeavor to advance the clinical research and market SYS6016 as soon as possible to create value for society and shareholders.
As of July 13, 2024, three RSV vaccines and one monoclonal antibody for infants (Beyfortus) were approved for use in the United States.

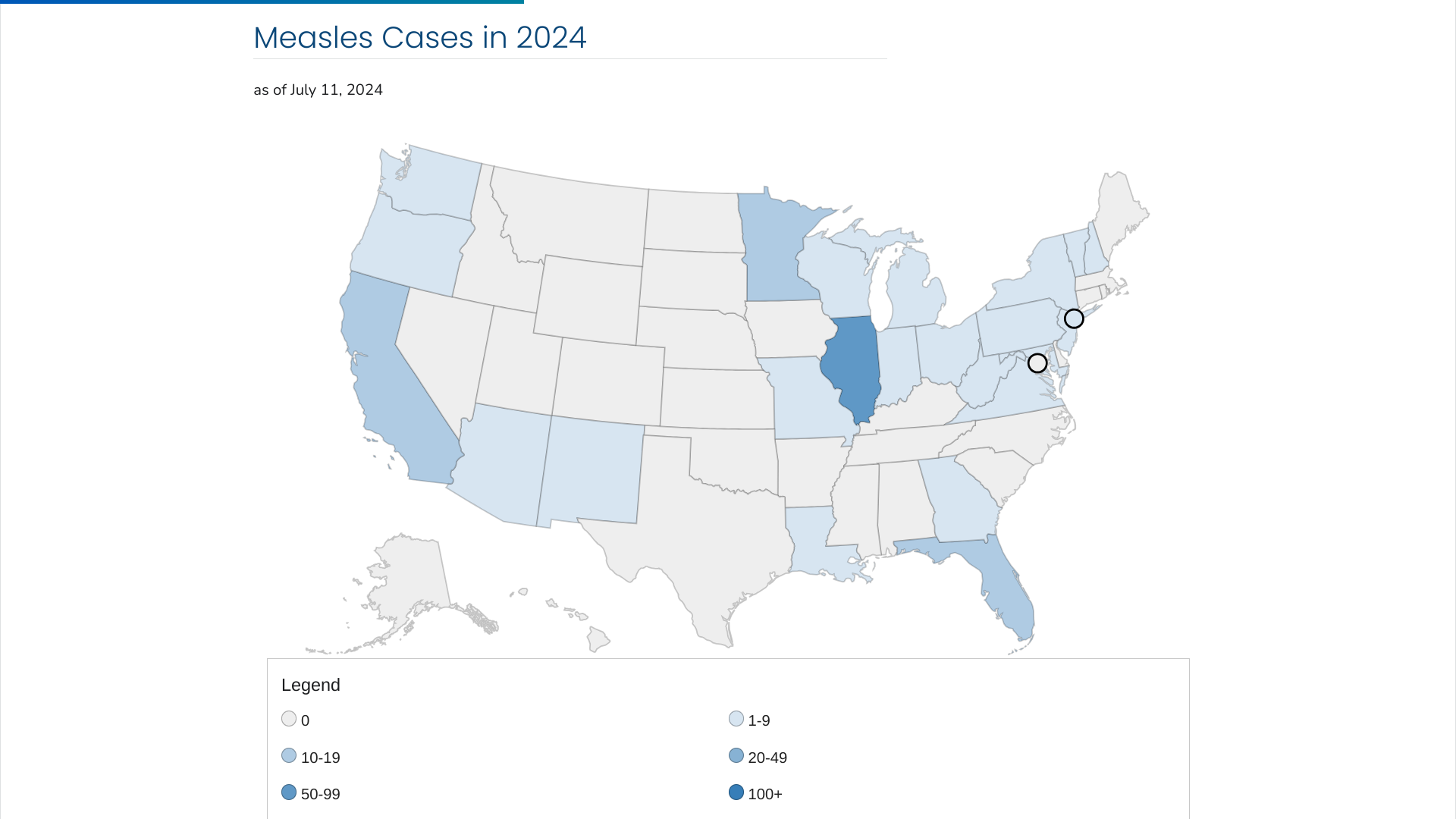
The Michigan Department of Health and Human Services (MDHHS) and the Macomb County Health Department today reported the sixth case of measles in the state in 2024.
The most recent case reported north of Detroit on July 12, 2024, had no known international travel association.
The U.S. Centers for Disease Control and Prevention (CDC) recently warned about possible travel-related measles outbreaks amid a global rise in cases.
On July 11, 2024, the CDC confirmed 167 cases have been reported this year, far outpacing the 58 measles cases reported in 2023.
In 2024, 84% of measles cases were in unvaccinated people or had an unknown vaccination status.
Throughout Michigan, pharmacies and clinics are offering various measles-preventing vaccines.
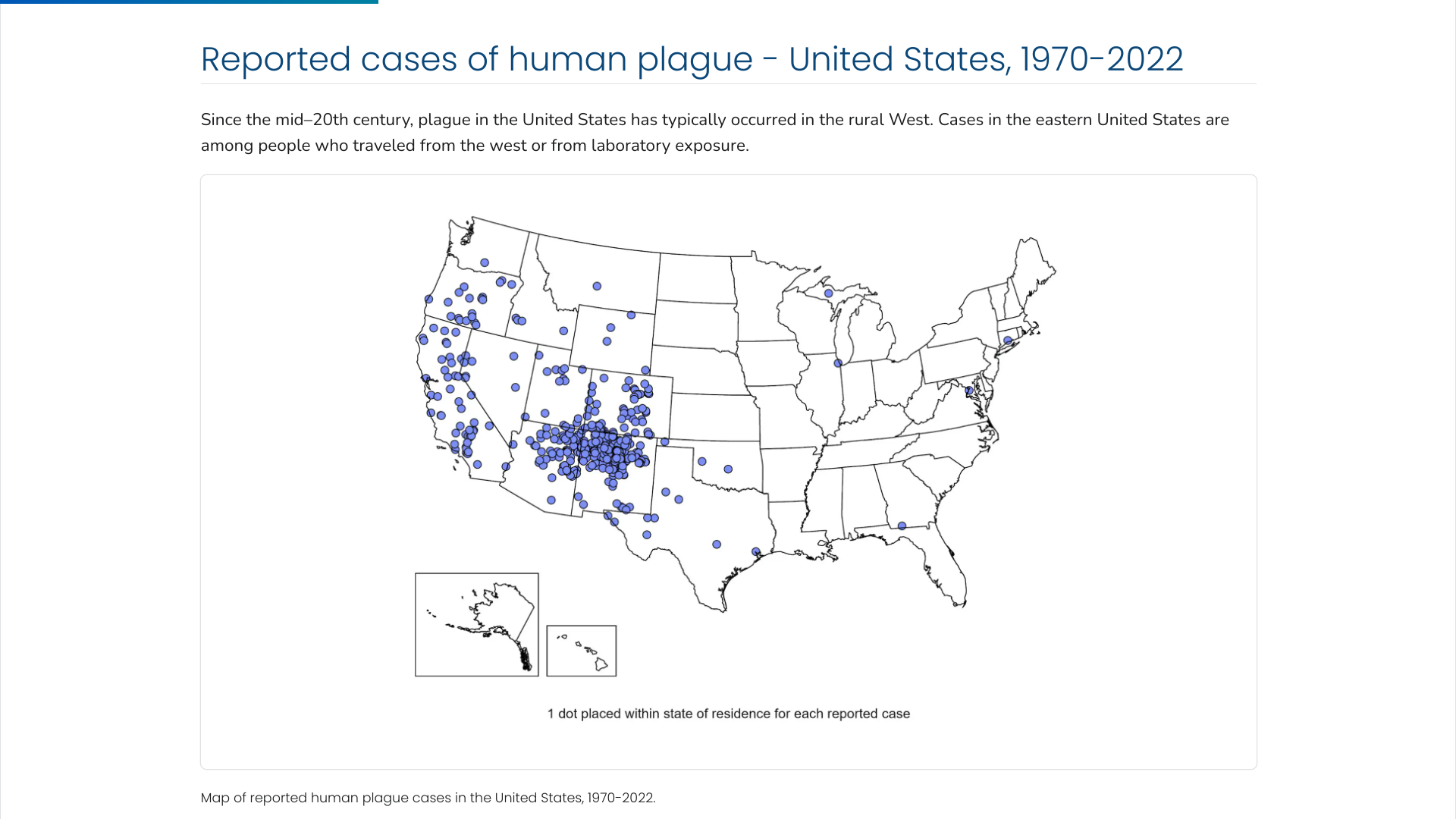
The U.S. Centers for Disease Control and Prevention (CDC) says the Plague was first introduced into the United States in 1900. The plague bacterium (Yersinia pestis) is transmitted by fleas and cycles naturally among wild rodents.
Over the decades, the Plague spread from urban rats to rural rodent species and became entrenched in many areas of the western U.S.
Almost all of the cases reported in the last 20 years have occurred among people living in small towns and villages or agricultural areas rather than in larger towns and cities, says the CDC.
As of 2024, the CDC estimates that seven human cases of Plague occur in the U.S. each year.
Recent plague cases include the Pueblo Department of Public Health and Environment confirming a human case of Plague in a Pueblo County resident on July 9, 2024.
And in February 2024, health officials in Oregon reported a case of bubonic Plague in a resident who they said likely contracted it from a pet cat.
Globally, the most human plague cases since the 1990s have occurred in Africa.
From a prevention perspective, plague vaccines are no longer available in the U.S. However, plague vaccine candidates are in development but are not expected to be commercially available in the immediate future.
In March 2023, the first mRNA-based, lipid nanoparticle vaccine was found effective against lethal bacteria in mice.
As numerous vaccine manufacturers strive to produce improved flu shot options, innovative influenza vaccine candidates post positive results in 2024.
Intranasal vaccines are known to provide a wall of defense at the site of infection, helping prevent influenza viruses from entering the nasal mucosa.
FluGen, Inc., today announced the results of its study comparing the coadministration of intranasal M2SR and the high-dose flu shot in older adults ages 65-85.
Published in the journal Lancet Infectious Diseases on July 11, 2024, the study, funded by the U.S. Department of Defense, evaluated the safety, tolerability, and immunological response to FluGen’s investigational supra-seasonal, live, single-replication, intranasal influenza vaccine when administered with Fluzone High Dose inactivated influenza vaccine.
These researchers concluded the H3N2 M2SR vaccine coadministered with Fluzone HD in older adults was well tolerated and provided enhanced immunogenicity compared with Fluzone HD administered alone.
This finding suggests the potential for improved influenza vaccination efficacy in this age group.
“The idea of delivering two vaccines in one sitting has become widely accepted,” said Paul Radspinner, President and Chief Executive Officer of FluGen, in a press release on July 12, 2024.
“Imagine being at your local pharmacy for your annual flu shot and also receiving a quick nasal spray that would greatly enhance your chances not only of becoming seriously ill but of being infected at all. This combination solution could have a tremendous impact on the health of older adults."
Radspinner went on to discuss the possible impact on influenza pandemic protection. “If H5N1 or any other mutating influenza strain were to begin infecting millions of people, imagine the benefits of combining an intranasal vaccine, which could stop most infections from occurring, with a strong antibody-based vaccine shot."
"The impact on human health could be unequaled in our history.”
As of July 2024, new trivalent influenza vaccines, such as Flucelvax, had been shipped to pharmacies before the 2024-2025 flu season launches in the United States.

Tiba Biotech LLC today announced its new partnership with the Biomedical Advanced Research and Development Authority (BARDA) through the initiation of an EZ-BAA contract to develop groundbreaking therapeutics against influenza.
The U.S. $749,999 BARDA contract supports early-stage therapeutic platform development for the Flexible and Strategic Therapeutics program.
This BARDA initiative aims to advance cutting-edge therapeutic platform technologies, such as RNA-mediated interference, which could be rapidly deployed in response to emerging viral threats. The therapeutic will target the highly conserved viral nucleoprotein and will be delivered via Tiba’s RNABLTM platform.
Tiba Biotech’s initial focus will be developing a prototype RNAi-based therapeutic for H1N1 influenza (swine flu), a type of influenza A virus.
Every year, there are rare, sporadic human infections with influenza viruses that usually circulate in pigs and not people, says the U.S. CDC.
On June 28, 2024, the CDC reported the second and third U.S. human infections in 2024.
This initiative is a natural extension of Tiba’s ongoing work combating influenza pandemic threats, most notably in the form of a novel multi-antigen mRNA-based H7N9 flu vaccine funded under a Phase II SBIR award from the National Institutes of Health and ongoing collaborations with the Collaborative Influenza Vaccine Innovation Centers.
Tiba was also recently accepted into BLUE KNIGHT™, a joint initiative between Johnson & Johnson Innovation—JLABS and BARDA. The initiative aims to harness innovation to combat future known and unknown health threats.



