Search API
As the global Chikungunya outbreak continues in July 2024, a second vaccine may soon become available.
This is essential news since the World Health Organization recently confirmed the mosquito-borne viral Chikungunya virus was identified in nearly 115 countries in 2024, primarily in the Region of the Americas.
Bavarian Nordic A/S today announced that the European Medicines Agency (EMA) has validated the marketing authorization application (MAA) submitted in June 2024 for CHIKV VLP, the Company’s vaccine candidate for immunization to prevent disease caused by chikungunya virus infection in individuals 12 years of age and older.
Validation of the MAA begins the EMA’s centralized review procedure under accelerated assessment.
The accelerated assessment, granted by EMA’s Committee for Medicinal Products for Human Use in February 2024, aims to reduce the timeframe for the CHMP to review an MAA. This could support the European Commission's vaccine approval in the first half of 2025.
Bavarian Nordic also completed the submission of a Biologics License Application for the CHIKV VLP vaccine to the U.S. Food and Drug Administration (FDA) in June 2024, potentially also supporting the approval of the vaccine in the first half of 2025.
CHIKV VLP is an adjuvanted VLP-based vaccine candidate for active immunization to prevent disease caused by CHIKV infection.
“The MAA submission and review marks a pivotal milestone for Bavarian Nordic in 2024....,” said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release on July 18, 2024.
In November 2023, the U.S. FDA approved Valneva SE's monovalent, single-dose, live-attenuated IXCHIQ® Chikungunya vaccine. It has been available in the U.S. since the beginning of 2024.
According to an email from an Administration for Strategic Preparedness and Response spokesperson on July 15, 2024, the first H5N1 avian influenza vaccine doses are scheduled to roll off the line next week, with the remaining doses following through August 2024.'
Other steps besides filling and finishing the vaccine, such as policy and regulatory decisions, must occur before the vaccine is released for public use.
Avian influenza vaccination has not been recommended for any population segment, and the U.S. government continues to monitor the situation.
Currently, the CDC evaluates the overall risk to human health as low.
On June 27, 2024, the U.S. Centers for Disease Control and Prevention confirmed during its vaccine committee meeting that an avian vaccination program was inactive.
As of July 17, 2024, the U.S. and European (Finland) governments have approved various avian influenza vaccines and recently awarded millions of dollars in vaccine candidate development contracts. Previous U.S. FDA-approved avian vaccines have reported measurable side effects.

Anixa Biosciences, Inc. today announced that its collaborator, Cleveland Clinic, has received a “Decision to Grant” notice from the Japan Patent Office for the patent application titled “Vaccine Adjuvants and Formulations.”
"This new Japanese patent extends the claims for this novel breast cancer vaccine technology to an additional geographic region, beyond the U.S. and European patents previously awarded," stated Anixa Chairman and CEO Dr. Amit Kumar in a press release on July 17, 2024.
Cleveland Clinic exclusively licensed this technology to Anixa Biosciences.
Cleveland Clinic researchers have identified a protein called alpha-lactalbumin that is present in healthy breast tissue only when a woman is lactating and disappears when she stops nursing her child.
Alpha-lactalbumin is never present on any other cell in the body. However, it does show up in many types of breast cancer, including an aggressive and deadly form of the disease known as Triple Negative Breast Cancer.
In addition, Cleveland Clinic researchers have identified that the extracellular domain of anti-Mullerian hormone receptor II (AMHR2-ED) is expressed in normal ovaries and nowhere else in the body, ceasing after menopause. However, this protein is also expressed in cancerous ovary cells.
By developing vaccines targeting alpha-lactalbumin and AMHR2-ED, the immune system can destroy breast and ovarian cancer cells, respectively, as they arise and ultimately prevent tumors from forming.
Following the positive clinical data seen in the breast cancer vaccine Phase 1 clinical trial, in collaboration with Cleveland Clinic, a cancer vaccine discovery program utilizing the same mechanism as breast and ovarian cancer vaccines to develop additional cancer vaccines to address many intractable cancers, including high-incidence malignancies in lung, colon, and prostate.

Diakonos Oncology Corporation announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for the company’s unique dendritic cell vaccine (DCV) for pancreatic ductal adenocarcinoma.
On July 15, 2024, the Houston-based company confirmed that DCVs are made with a patient’s dendritic cells and a tumor sample.
These highly differentiated double-loaded dendritic cell vaccines activate robust cytotoxic TH1 cell signaling pathways that initiate a natural immune response to target and eliminate cancer cells. This is achieved without any genetic modification of the patient’s immune cells, which greatly simplifies the manufacturing process and significantly reduces costs compared to leading cell therapy approaches.
“This second FDA Fast Track designation of our autologous dendritic cell vaccines for pancreatic cancer is another acknowledgment of the incredible potential of this innovative immunotherapy for treating the most deadly cancers,” said Mike Wicks, Diakonos CEO, in a press release.
Pancreatic ductal adenocarcinoma is the most common pancreatic cancer. In 2024, an estimated 51,750 people will die, and 66,440 will be newly diagnosed.
FDA Fast Track designation is intended to speed the development and review of drugs that show early clinical promise in treating severe or life-threatening conditions.

During today's U.C. CDC Clinician Outreach and Communication Activity call, the presentation offered insights regarding the multi-year Highly Pathogenic Avian Influenza (HPAI) (H5N1) virus outbreak in the United States.
As of July 16, 2024, the HAPI virus is widespread among wild birds and continues to cause outbreaks in poultry and spillover to mammals, including dairy cattle.
To date, three human cases of HPAI A(H5N1) virus infection have been identified in dairy farm workers in the U.S.
The CDC team wrote, 'The risk to the public from HPAI A(H5N1) viruses is low. However, people with job-related or recreational exposure to infected birds or animals, including dairy cattle, are at greater risk of HPAI A(H5N1) virus infection.
As of July 2024, the U.S. government has invested hundreds of millions of dollars in funding avian influenza vaccines and clinical candidates that could be deployed during a pandemic.

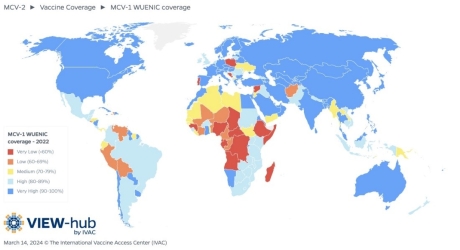
New data published by the World Health Organization (WHO) highlights brighter spots in global immunization coverage. On July 15, 2024, the WHO wrote the steady introduction of new and under-utilized vaccines, including vaccines targeting human papillomavirus (HPV), has produced positive results.
About 85% of people will get an HPV infection in their lifetime.
For example, the share of adolescent girls globally who received at least one dose of the HPV vaccine, which protects against cervical cancer, increased from 20% in 2022 to 27% in 2023.
The use of the single-dose HPV vaccine schedule also helped boost vaccine coverage.
Strong introductions in countries such as Bangladesh, Indonesia, and Nigeria primarily drove this increase.
However, HPV vaccine coverage is well below the 90% target to eliminate cervical cancer as a public health problem, reaching only 56% of adolescent girls in high-income countries and 23% in low- and middle-income countries.
"The HPV vaccine is one of the most impactful vaccines in Gavi’s portfolio, and it is incredibly heartening that it is now reaching more girls than ever before,” said Dr. Sania Nishtar, CEO of Gavi, the Vaccine Alliance, in a WHO press release.
“With vaccines now available to over 50% of eligible girls in African countries, we have much work to be done, but today, we can see we have a clear pathway to eliminating this terrible disease.”
New insights suggest innovative marketing programs are needed to expand HPV vaccine uptake.
A recent poll of over 400,000 users of UNICEF’s digital platform for young people, U-Report, revealed that over 75% are unaware or unsure of what HPV is, underscoring the need for better vaccine accessibility and public awareness.
When informed about the virus, its link to cancers, and the existence of a vaccine, 52% of respondents indicated they want to receive the HPV vaccine but are hindered by financial constraints (41%) and lack of availability (34%).
As of July 2024, various HPV vaccines were available worldwide.
In the United States, the CDC recommends two doses of HPV vaccine for all adolescents at age 11 or 12 years.

New data published today by the World Health Organization (WHO) revealed that over the last five years, measles outbreaks have hit 103 countries.
In 2023, nearly 35 million children had no or only partial measles protection.
In July 2024, the U.S. CDC listed the top ten international measles outbreaks led by Azerbaijan, Kazakhstan, Iraq, and India. In the United States, the CDC reported 167 measles cases in 24 jurisdictions this year.
On July 15, 2024, the WHO confirmed that only 83% of children worldwide received their first dose of a measles vaccine through routine health services, while the number of children receiving their second dose reached 74%.
These WHO figures fall short of the 95% coverage needed to prevent measles outbreaks.
“Measles outbreaks are the canary in the coal mine, exposing and exploiting gaps in immunization and hitting the most vulnerable first,” said Dr. Tedros Adhanom Ghebreyesus, WHO Director-General, in a press release.
“This is a solvable problem."
"Measles vaccine is cheap and can be delivered even in difficult places. WHO is committed to working with all our partners to support countries in closing these gaps and protecting the most at-risk children as quickly as possible.”
The CDC confirmed in 2024 that if you are unsure if you or your travel companions are fully protected against measles, schedule an appointment to see your clinician at least six weeks before traveling so that you have enough time to get vaccinated with an MMR vaccine.