Search API
About 10% of children in Gavi-supported countries do not receive a single dose of routine vaccines.
To reach these missing millions, Gavi announced on July 11, 2024, that the 5.0 Strategy intends to reduce the number of zero-dose children by 25% by 2025 and 50% by 2030.
The new strategy will focus on reaching the most marginalized by strengthening primary healthcare systems, building and sustaining community demand, addressing gender barriers, and using innovation to ensure immunization services reach these children.
Available data suggests the largest disruptions were concentrated in Q2 2020, with the majority of countries restoring routine immunization services in the second half of the year. Over 75% of under-immunised children are now zero-dose, heightening the risk of child deaths, disease outbreaks, and medical impoverishment.
In 2000, just 47% of children in lower-income countries received essential vaccines.
In Gavi-eligible countries, coverage of critical vaccines increased by three percentage points from 2015 to 2019, and the number of zero-dose children was reduced by 14%.
In 2019, coverage for the same countries reached 82% before sliding back to 78% due to the pandemic.
According to data published by the World Health Organization and UNICEF, in 2022, 20.5 million children in India missed out on one or more vaccines delivered through routine immunization services.
Gavi wrote, 'The importance of immunization reaching all children is paramount and ensures all children have an equal chance of being healthy and productive members of society.'
'Vaccination, because of its preventative nature, averts illness and provides particularly significant benefits to zero-dose communities which may lack access to affordable, quality curative care while being at higher risk of vaccine-preventable diseases.'
'Immunised children are also more likely to grow up healthy and enjoy their survival and development rights. Vaccinated children have higher cognitive abilities, miss school less and are in school for longer, and have better nutrition and education outcomes – all of which translates into better-earning potential and productivity as an adult.'

The U.K. Medicines and Healthcare products Regulatory Agency (MHRA) today approved an adapted Pfizer/BioNTech mRNA COVID-19 vaccine (Comirnaty) targeting the JN.1 COVID-19 subvariant.
On July 24, 2024, the MHRA announced four forms of this adapted Comirnaty JN.1 vaccine have been approved by the MHRA under the International Recognition Procedure after they were found to meet the U.K. regulator’s safety, quality, and effectiveness standards.
The MHRA confirmed that the vaccine administration differs between adults and children from infancy, depending on their age at the time of vaccination.
These forms are as follows:
Comirnaty JN.1 30 micrograms/dose dispersion for injection and Comirnaty JN.1 30 micrograms/dose dispersion for injection in a pre-filled syringe for use in adults.
Comirnaty JN.1 10 micrograms/dose dispersion for injection, single-dose vial for use in children from 5 to 11 years of age.
Comirnaty JN.1 3 micrograms/dose concentrate for dispersion for injection, 3-dose vial for use in infants and children from 6 months to 4 years of age.
If a patient experiences any Comirnaty-related side effects, they should talk to their doctor, pharmacist, or nurse. A full list of all side effects reported with this medicine is available in the patient information leaflet, which can be obtained from the pharmacy or the product information published on the MHRA website.

The International Journal of Infectious Diseases recently published a new study conducted by Columbia University that revealed a 140% surge in measles cases worldwide from 2010 to 2019 across 194 member countries of the World Health Organization.
In a Short Communication dated July 22, 2024, the researchers highlighted that decreasing vaccination rates in 59 of the 194 nations were attributed to socio-economic issues in some less developed countries and vaccine hesitancy in wealthier nations.
In July 2024, the U.S. Centers for Disease Control and Prevention (CDC) listed the top ten international measles outbreaks led by Azerbaijan, Kazakhstan, Iraq, and India. In total, the CDC listed 52 countries.
"Our analysis also suggests vaccine hesitancy may substantially contribute to the recent increases in measles incidence in wealthier countries. For instance, recent measles outbreaks in Europe and the United States have been linked to international travel and communities with prevailing vaccine skepticism,' wrote these researchers.
As of July 11, 2024, a total of 167 measles cases were reported by Arizona, California, Florida, Georgia, Illinois, Indiana, Louisiana, Maryland, Michigan, Minnesota, Missouri, New Hampshire, New Jersey, New Mexico, New York City, New York State, Ohio, Oregon, Pennsylvania, Vermont, Virginia, Washington, West Virginia, and Wisconsin.
The CDC reports 13 measles outbreaks in 2024, compared to 4 outbreaks reported in 2023.
Various U.S. FDA-approved measles vaccines are generally available at pharmacies in the United States.
Gilead Sciences, Inc. today announced full efficacy and safety results from its pivotal HIV-1 Phase 3 clinical trial.
Detailed data from the trial’s interim analysis announced in June 2024 showed that lenacapavir, the company’s twice-yearly injectable HIV-1 capsid inhibitor, demonstrated zero infections, 100% efficacy, and superiority to background HIV incidence for the investigational use of HIV prevention in cisgender women.
Lenacapavir also demonstrated superior prevention of HIV infections when compared with once-daily oral Truvada.
The new data provide details on the efficacy, safety, and tolerability of twice-yearly lenacapavir injections; drug adherence among trial participants, including poor levels of adherence to daily oral pre-exposure prophylaxis (PrEP) and high levels of adherence to lenacapavir; and demographic and behavioral characteristics of trial participants, including pregnant women and adolescents.
The data were published today in The New England Journal of Medicine.
“These stellar results show that twice-yearly lenacapavir for PrEP, if approved, could offer a highly effective, tolerable and discreet choice that could potentially improve PrEP uptake and persistence, helping us to reduce HIV in cisgender women globally,” said Linda-Gail Bekker, MBChB, DTM&H, DCH, FCP(SA), PhD, Director of the Desmond Tutu HIV Center at the University of Cape Town, South Africa, and former President of the International AIDS Society, in a press release on July 24, 2024.
“PURPOSE 1 also sets a new standard for person-centered HIV prevention trials, demonstrating what can happen when a thoughtful scientific and community-focused trial design, a promising drug candidate, and an inclusive trial implementation plan come together.”
Gilead expects results in late 2024/early 2025 from the program’s other pivotal trial, PURPOSE 2, which is assessing twice-yearly lenacapavir for PrEP among men.
Currently, there are no cures for HIV or AIDS or preventive vaccines available.

The Committee on Immunization of Quebec (CIQ) today recommended using RSV (respiratory syncytial virus) vaccines, including AREXVY™, to prevent RSV among older adults at increased risk of severe outcomes from the virus.
Specifically, CIQ recommends vaccination for older adults living in residential and long-term care centers, intermediate resources, adults aged 75 and older living in private seniors’ residences, and those living in the community with chronic illnesses.
The CIQ recommendations for RSV vaccination follow those issued by the National Advisory Committee on Immunization, published earlier in July 2024.
Marni Freeman, Country Medical Director, GSK Canada, said in a press release on July 24, 2024, “As our immune system ages, we all become more vulnerable to severe consequences of RSV disease."
"Older adults who are immunocompromised or suffer from underlying medical conditions, such as chronic heart or lung disease, are at an even greater risk.
"The CIQ recommendation reflects the important role AREXVY can play in reducing the incidence and overall burden of respiratory syncytial virus among Quebec’s older adult population, and we look forward to collaborating with public health officials, healthcare professionals, and payers to ensure optimal vaccine access in the province.”
AREXVY was approved in Canada in August 2023 and is indicated for preventing lower respiratory tract disease caused by RSV in individuals 60 and older.
This vaccine (May 2023), two other RSV vaccines, and one monoclonal antibody for infants have been approved for use in the United States.
RSV is a common, contagious virus that affects the lungs and respiratory airways. For most people, the virus causes cold-like symptoms. Still, for older adults and adults with certain health conditions, it can lead to more serious infections and complications such as pneumonia, hospitalization, and even death, says the CIQ.

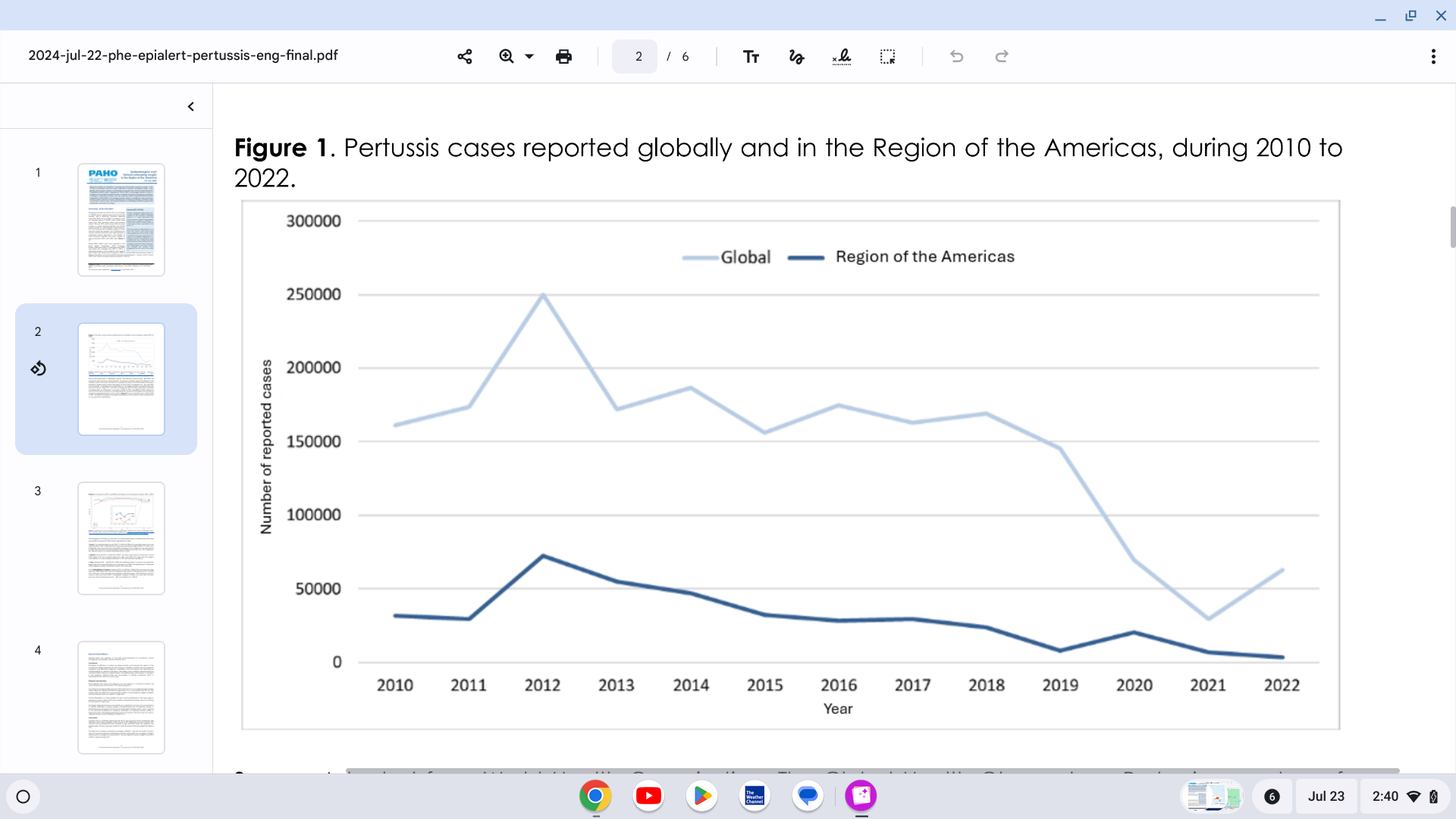
The Pan American Health Organization (PAHO) reported yesterday a significant increase in pertussis (whooping cough) cases in the Region of the Americas.
On July 22, 2024, the PAHO confirmed that 7,251 pertussis cases were reported in the United States in 2024, a 300% increase from last year.
Pertussis cases in Mexico are 242% higher than reported in 2023. Brazil and Peru are also reporting measurable case increases.
In the Region of the Americas, 2012 was the year with the highest number of cases reported during the decade, with 72,328 reported cases of pertussis. Since then, there has been a progressive annual decrease in the reported cases, reaching the lowest number reported in 2022, with 3,283 pertussis cases.
The first and third doses of diphtheria, tetanus, and pertussis vaccines (DTP1 and DTP3) are commonly used as tracers of immunization coverage. The coverage trend for both first and third doses has shown a significant decline.
The year 2021 was the lowest coverage year in the Region of the Americas compared with the previous 20 years. However, updated vaccine coverage data for 2023 reported a recovery of 90% for DTP1 and 88% for DTP3.
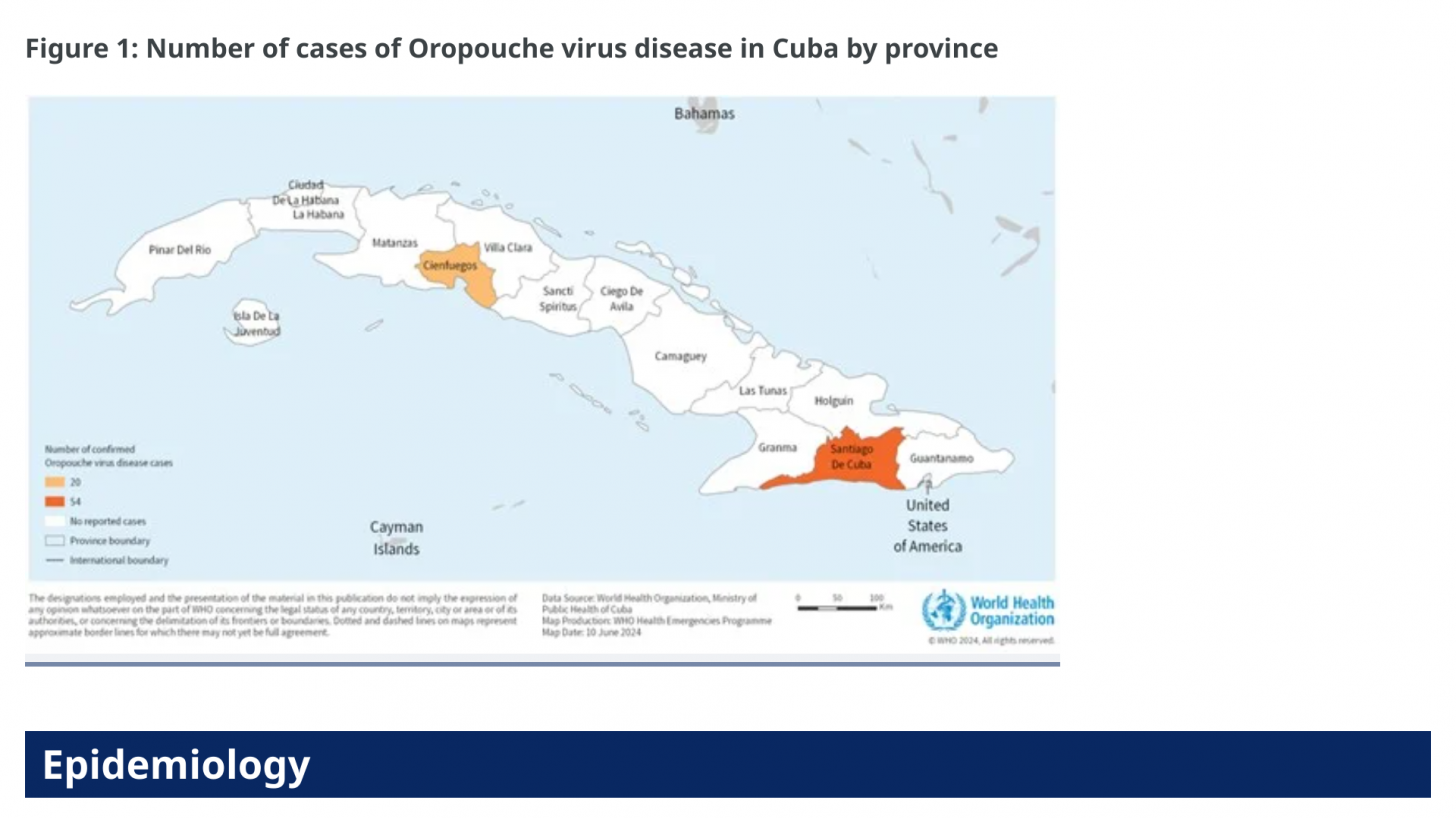
According to the European CDC, Italy and Spain each reported three confirmed cases of Oropouche virus disease in travelers returning from Cuba.
The ECDC stated on July 12, 2024, that the likelihood of secondary transmission of the Oropouche virus within continental Europe is considered very low due to the absence of known competent vectors, midges (small flies), and mosquitoes, commonly found in the Region of the Americas.
To alert international travelers, the U.S. CDC issued a Level 1 Travel Health Advisory in June 2024. This advisory stated that people should seek medical care if they develop high fever, headache, muscle aches, stiff joints, nausea, vomiting, chills, or sensitivity to light during or after travel.
As of July 23, 2024, no U.S. FDA-approved vaccines for Oropouche virus disease exist.
Globally, respiratory syncytial virus (RSV) is the leading cause of hospitalization for healthy infants under a year old and causes an estimated 101,000 deaths a year in children under five.
To address this significant health risk, Merck today announced positive topline results from its Phase 2b/3 clinical trial evaluating clesrovimab (MK-1654), the company’s investigational prophylactic monoclonal antibody (mAb) designed to protect infants from respiratory syncytial virus (RSV) disease.
Clesrovimab met its primary safety and efficacy endpoints in the trial, including reducing medically attended lower respiratory infections caused by RSV through Day 150.
Clesrovimab is being studied in infants (pre-term and full-term) to provide rapid, durable protection through their first RSV season with a single, fixed-dose administration.
“RSV is highly contagious and can cause inflammation in the airways of infants, leading to difficulty breathing. As a widespread illness globally, RSV is the leading cause of hospitalization for healthy infants,” said Dr. Paula Annunziato, senior vice president of infectious diseases and vaccines, Global Clinical Development, Merck Research Laboratories, in a press release on July 23, 2024.
“We are encouraged by these findings and look forward to working with regulators to provide a new option to help address the impact of RSV on infants and their families."
For the 2024-2025 RSV season, the U.S. FDA-approved Beyfortus™ (Nirsevimab) mAb offers passive immunization to prevent lower respiratory tract infections caused by the RSV to infants experiencing their first or second RSV season and those with congenital heart disease or chronic lung disease.
Additionally, one vaccine has been approved for pregnant women, which offers RSV protection to newborns.


