Search API
Harris County Public Health’s (HCPH) Mosquito and Vector Control Division today reported a significant increase in West Nile virus (WNV), which is the leading cause of mosquito-borne disease in the continental United States.
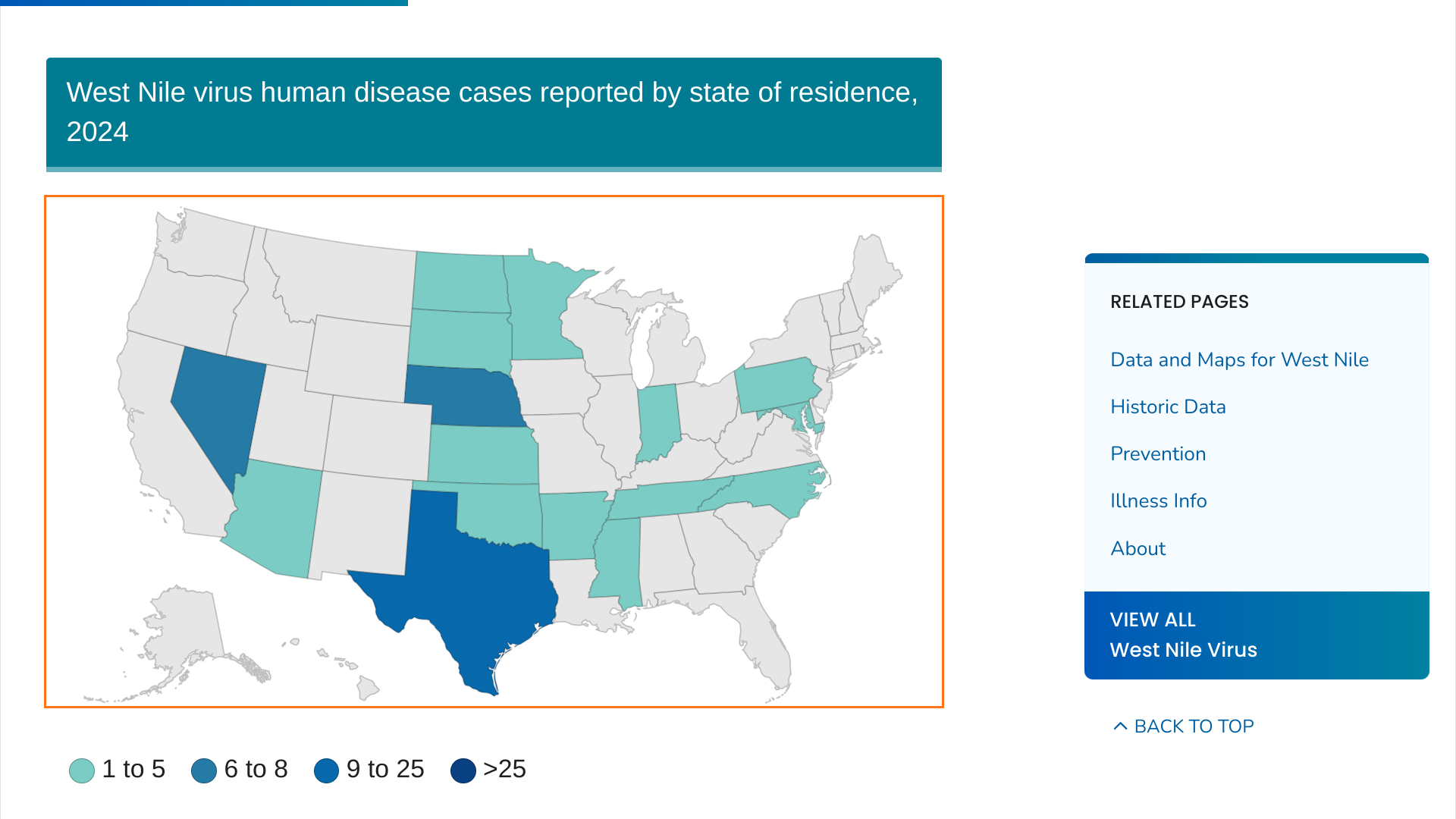
On July 26, 2024, seven human cases of WNV were reported to HCPH in unincorporated Harris County (outside the City of Houston).
Additionally, 520 positive mosquito samples were identified across 168 of its 268 operational areas in Harris County, which has a population of about 4.9 million.
HCPH urges residents to protect themselves and their loved ones against this mosquito-transmitted illness.
As of July 23, 2024, the U.S. CDC reported 45 WBV disease cases and 24 WNV neuroinvasive disease cases from 19 states this year.
Furthermore, the CDC says there are no vaccines or medicines to prevent WNV disease.
Several vaccine candidates, including live attenuated chimeric, DNA (first and second generation), recombinant subunit, and inactivated whole-virus vaccines have been the subject of human clinical studies.
The Minnesota Department of Health (MDH) recently confirmed three additional measles cases in unvaccinated children in Anoka, Hennepin, and Ramsey counties.
MDH stated that based on current information, these cases are not directly linked and have not traveled, so there is concern for the possible spread of measles in the community.
As of July 26, 2024, Minnesota has confirmed 15 measles cases in 2024, and is an increase compared to other years. All the cases have occurred among unvaccinated children.
Minnesota isn’t the only state to have seen an increase in measles cases. Just to the south, Chicago, Illinois, reported a significant outbreak (64 cases) this year.
MDH is working with local health departments and other locations to notify people who may have been exposed directly. However, health officials note that anyone not vaccinated against measles could be at risk and should watch for symptoms of measles.
“Measles spreads easily, and it finds those who are vulnerable,” said Jessica Hancock-Allen, infectious disease division director at MDH, in a press release.
“That is why families need to ensure their children are up to date on their immunizations to protect them from this potentially serious disease.”
"The best way to prevent measles is through immunization."
Measles vaccines are generally available at clinics and pharmacies throughout the U.S.

As flu shots arrive in local pharmacies next month, Canadians will have different vaccines to choose which is best for their needs.
To assist this decision process, the Canadian National Advisory Committee on Immunization’s (NACI) annual Statement on Seasonal Influenza Vaccines for 2024-2025 recommends Fluzone® High-Dose Quadrivalent among the preferential influenza vaccines over standard-dose influenza vaccines.
According to NACI on July 26, 2024, Fluzone® High-Dose Quadrivalent has the most substantial body of supporting evidence among preferentially recommended vaccines for adults 65 years of age and older.
Dr. Angel Chu MD, FRCPC, Infectious disease specialist, Clinical Assistant Professor, University of Calgary, STI Clinic Calgary, and Vice-Chair of Immunize Canada, commented in a press release, “In the newest NACI statement, Fluzone® High-Dose continues to be recommended for adults 65 years of age and older. NACI also recognizes Fluzone® High-Dose has the most substantial body of supporting evidence among flu vaccines for seniors.”
Influenza can cause mild to severe illness. Some populations, especially young children and adults 65 and older, are at a higher risk for serious influenza complications.
Sanofi says vaccination is the most effective way to prevent influenza and its complications.
Earlier this year, the WHO recommended that trivalent vaccines be deployed during the 2024-2025 northern hemisphere influenza season.
On June 27, 2024, the U.S. Centers for Disease Control and Prevention vaccine committee meeting included presentations focused on Considerations and Proposed Recommendations for the 2024-25 Influenza Season in the United States. Physicians, nurses, and pharmacists can offer patients up to nine different influenza vaccines for the 2024 - 2025 flu season.

The Ontario Ministry of Health has announced the first publicly funded universal program with Beyfortus® (nirsevimab) for all newborns and infants born in 2024 and through the 2024-2025 respiratory syncytial virus (RSV) season in the Northern Hemisphere.
Beyfortus single-dose administration can be timed to the start of the RSV season.
RSV is a common respiratory virus that often impacts children and can lead to lung infections such as bronchiolitis and pneumonia.
As of July 25, 2024, this new passive immunization program also includes some high-risk children up to 24 months old.
Beyfortus is administered directly to newborns and infants and offers rapid protection via an antibody without requiring immune system activation.
Delphine Lansac, General Manager, Vaccines, Sanofi Canada. commented in a press release on July 25, 2024, "Today's announcement by the Government of Ontario is a significant milestone. Providing universal access to Beyfortus® to help protect all infants in Ontario means that parents can focus on the joys of a new baby and worry less about experiencing a severe RSV infection."
"This new program builds on our 110-year heritage as a committed partner supporting public health in Canada. Our objective continues to be protecting the health of Canadians with innovative solutions and introducing Beyfortus® is a step forward to protect babies and make a positive difference for families and the healthcare system."
Health Canada issued a Notice of Compliance for Beyfortus in April 2023. Additionally, the single-dose, extended half-life monoclonal antibody was approved by the U.S. FDA in July 2023 and the European Union in October 2022.
According to media reports, access to Beyfortus is expected to meet demand in the United States during the second half of 2024.
AstraZeneca, responsible for Beyfortus manufacturing, confirmed regulatory applications for two additional filling lines have been submitted to health authorities to expand supply. This production expansion is anticipated to augment capacity compared to the one licensed line.

Bavarian Nordic A/S today announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) had recommended the approval of a type II variation for IMVANEX® (MVA-BN, JYNNEOS®) smallpox and mpox vaccine.
This EMA recommendation includes real-world effectiveness data from the use of the vaccine during the global 2022 mpox outbreak in the marketing authorization.
In real-world studies, vaccine effectiveness against mpox disease was demonstrated at least 14 days after vaccination, with adjusted vaccine effectiveness estimates ranging from 35% (95% CI, -2-59) to 89% (95% CI, 76-95) after one MVA-BN dose and from 66% (95% CI, 47-78) to 90% (95% CI, 86-92) after two MVA-BN doses.
Furthermore, in a surveillance study, MVA-BN reduced the risks of mpox-related hospitalization.
Compared with unvaccinated mpox patients, the odds of hospitalization were 0.27 (95% CI, 0.08-0.65) after one MVA-BN dose and 0.20 (95% CI, 0.01-0.90) after two MVA-BN doses. The estimated relative risk reduction was 73% after one MVA-BN dose and 80% after two MVA-BN doses.
“The 2022 global mpox outbreak provided an opportunity to assess the effectiveness of our vaccine in at-risk populations across different geographies, both before and after exposure to the mpox virus, and we are pleased to receive the recommendation to include real-life data in our marketing authorization in Europe, which confirm a high effectiveness of up to 90% after two doses of the vaccine as recommended by the authorities. It is furthermore encouraging that data show the vaccine to reduce the risk of hospitalizations significantly, thus confirming our vaccine as an important and versatile tool in the fight against mpox globally,” said Paul Chaplin, President and Chief Executive Officer of Bavarian Nordic, in a press release on July 26, 2024.
As of July 2024, the JYNNEOS vaccine is commercially available in the United States.

Sanofi today announced strong performance and increasing sales growth. In Q2 2024, Sanofi's sales were €10,745 million, up 10.2%.
In a press release on July 25, 2024, Paul Hudson, CEO, commented, “We are continuing our strong performance in 2024 and delivered broad-based, double-digit sales growth in the second quarter."
However, its very popular single-dose, extended half-life monoclonal antibody Beyfortus™ (Nirsevimab) offers passive immunization to prevent lower respiratory tract infections caused by the respiratory syncytial virus (RSV) to newborns and infants experiencing slow sales growth.
Beyfortus sales were limited to €18 million (USD $19.6), reflecting the global vaccine seasonality towards the second half-year. In the first quarter of 2024, Beyfortus produced €200 in sales.
In collaboration with AstraZeneca, responsible for Beyfortus manufacturing, the regulatory applications for two additional filling lines have been submitted to health authorities to expand supply for the Northern Hemisphere ahead of the 2024 - 2025 RSV season.
This production expansion is anticipated to augment capacity compared to the one licensed line.
According to media reports, access to Beyfortus is expected to meet demand in the United States during the second half of 2024.