Search API
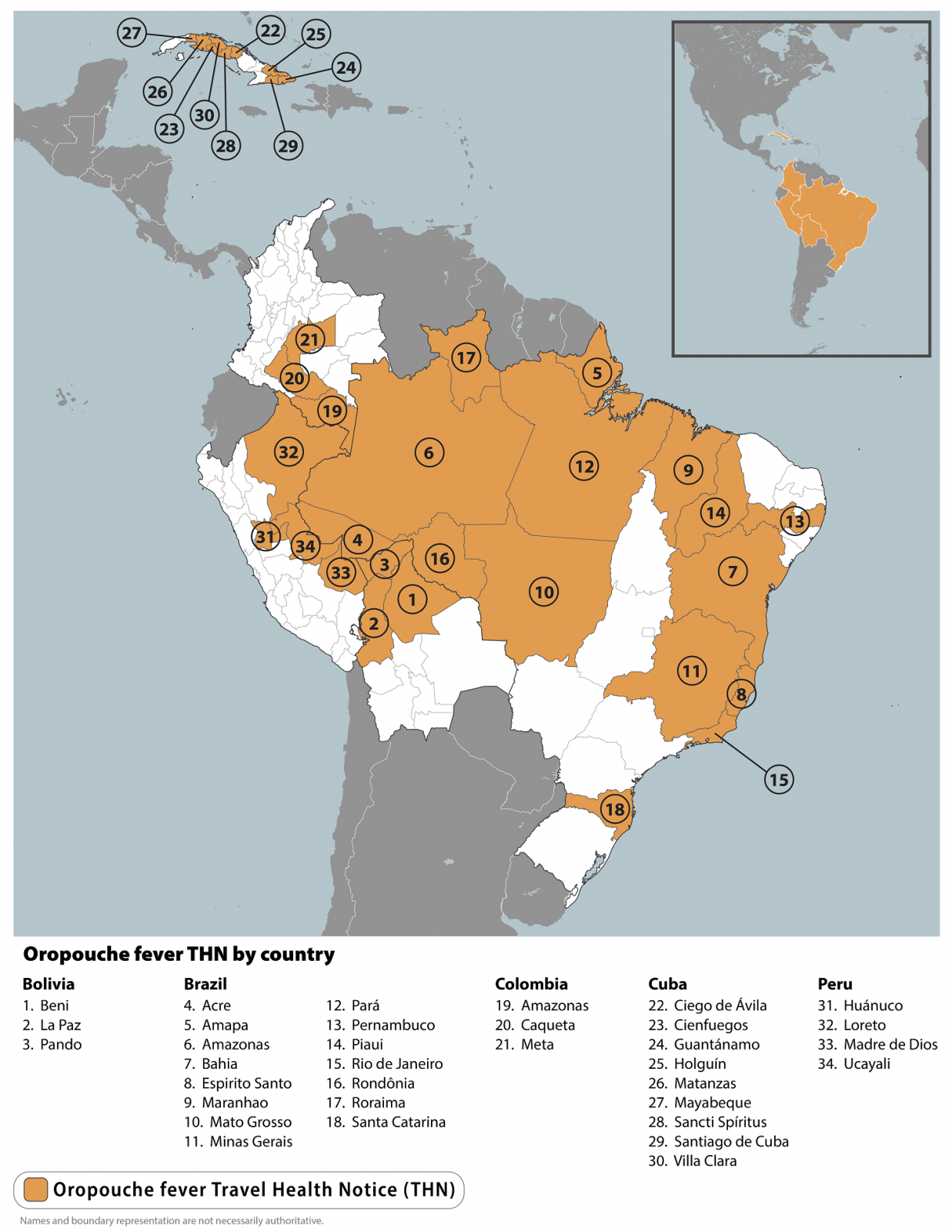
The U.S. Centers for Disease Control and Prevention (CDC) recently issued a Level 1 Travel Health Advisory confirming outbreaks of Oropouche fever in parts of Brazil, Bolivia, Colombia, Peru, and Cuba.
On August 1, 2024, the CDC reported that 34 countries had reported 8,078 Oropouche fever cases this year.
Local transmission has also been documented in ten non-Amazonian states, some without previous cases reported. In Brazil, 7,284 cases were confirmed, mainly in the Amazon region.
Travelers to affected areas should avoid infected midges (small flies) and mosquitoes as they spread this disease, which is often mistaken for dengue. The CDC says travelers should seek medical care if they develop high fever, headache, muscle aches, stiff joints, nausea, vomiting, chills, or sensitivity to light during or after travel.
According to reports, Oropouche fever can be passed between a pregnant woman and an unborn child.
As of July 30, 2024, five potential cases of vertical transmission have been identified in Brazil: four cases of stillbirth and one case of spontaneous abortion.
On July 17, 2024, the Pan American Health Organization (PAHO) said it is not clear if infection with the Oropouche virus caused adverse health outcomes for the fetuses.
The CDC is working with PAHO and other international partners to learn more about the potential risks of Oropouche during pregnancy.
As of August 6, 2024, there are no approved vaccines to protect people from this disease.
ImmunityBio, Inc., today announced the opening of the National Cancer Institute-sponsored Phase 1/2 QUILT 502 clinical trial, which will study ANKTIVA® together with the investigational AdHER2DC vaccine in individuals with HER2-expressing endometrial cancer.
It marks the latest trial involving ANKTIVA, the company’s IL-15 superagonist immune enhancer. The aim of the trial is to evaluate ANKTIVA as an agent to replace the short-term activity of checkpoint inhibitor immunotherapies with long-term effectiveness.
Endometrial cancer is the most common gynecological cancer in the U.S. and affects more than 65,000 women each year, with incidence peaking around 50-60 years of age. The 5-year overall survival rate in patients with metastasis is around 20 percent; treatment options after the second-line treatment are limited.
The AdHER2DC vaccine targets the HER2 protein, which is elevated in 30% of patients with endometrial cancer and in more than 50% of high-risk subtypes. The single agent AdHER2DC demonstrated a safety profile and immunogenicity in a phase 1 clinical trial.
The U.S. FDA recently approved ANKTIVA for BCG-unresponsive non-muscle invasive bladder cancer CIS with or without papillary tumors.
“We are pleased to partner with the NCI on this important cancer control study involving ANKTIVA, which has demonstrated in clinical trials that activation of memory T cells may help deliver long-duration responses well beyond that of checkpoint inhibitors alone,” said Patrick Soon-Shiong, M.D., Executive Chairman and Global Chief Scientific and Medical Officer at ImmunityBio, in a press release on August 6, 2024.
“We are hopeful that the AdHER2DC investigational vaccine plus ANKTIVA will ‘rescue’ the checkpoint inhibitor pembrolizumab and kinase inhibitor lenvatinib and lead to an improved response compared with the current standard of care in this high-risk population.”
Phase 1 of the open-label, two-arm Phase 1/2 study will determine the recommended dose of pembrolizumab, lenvatinib, ANKTIVA, and AdHER2DC in participants with HER2-positive endometrial cancer.
The Phase 2 portion of the study will assess the efficacy of the combination of pembrolizumab, lenvatinib, ANKTIVA, and the AdHER2DC vaccine in qualified participants as determined by the proportion of participants without disease progression at six months. The study will enroll 60 subjects and is expected to be completed in 2026.
ImmunityBio says these studies, along with the recent approval of ANKTIVA for bladder cancer, signal the advent of the era of cytokines as the next generation of immunotherapies.
To combat one of the most lethal forms of pediatric brain cancer, UCLA Health Jonsson Comprehensive Cancer Center researchers are launching a first-of-its-kind clinical trial to evaluate the safety and effectiveness of a cancer vaccine candidate targeting H3 G34-mutant diffuse hemispheric glioma.
This highly aggressive brain tumor is typically found in adolescents and young adults.
A particular mutation of the H3-3A gene primarily characterizes this type of brain tumor. This mutation leads to significant disruptions in RNA processing, with wide-ranging influences on cancer behavior and response to treatment.
The vaccine candidate developed at UCLA targets these tumor genetic mutations. UCLA Health is the only center investigating immunotherapy for this type of glioma.
“Despite aggressive treatments, this type of brain tumor evades current therapies with shocking efficiency,” said Dr. Anthony Wang, director of the Pediatric Brain Tumor Program at UCLA Health and the principal investigator of the trial, in a press release on August 5, 2024.
“These cancers show a host of escape pathways, allowing small populations of cells to survive initial treatment and to adapt. The data from our pre-clinical studies makes us hopeful that an active, targeted cancer vaccine will be able to adapt with the tumor to eliminate cancer cells more effectively.”
The vaccine works by arming a patient’s dendritic cells, the most efficient activator of the body’s immune system, to target products of the altered RNA regulation that defines this cancer type.
Once activated against these targets, the patient’s dendritic cells are injected back into the patient.
Dendritic cell vaccination has already shown promise in treating some other forms of cancer, including glioblastoma, adding years of life for a subset of patients with a disease that often only has a lifespan of months.

Biopharmaceutical New Technologies (BioNTech) SE today announced about €807.8 million in losses in the second quarter of 2024. This negative report compares to $208 million during the same period in 2023. The increased operating loss was impacted by decreased demand for mRNA COVID-19 vaccines.
From a working capital perspective, the Company ended the second quarter of 2024 with €18.5 billion in cash, cash equivalents, and security investments.
Prof. Ugur Sahin, M.D., CEO, and Co-Founder of BioNTech, commented in a press release on August 5, 2024, “In addition, we have started commercializing variant-adapted COVID-19 vaccines for the upcoming season while accelerating our clinical development efforts to realize the full potential of our technologies."
On July 24, 2024, the United Kingdom’s Medicines and Healthcare products Regulatory Agency approved the companies’ Omicron JN.1-adapted vaccine.
Sahin added, "We are making progress towards our goal of becoming a company with marketed medicines for cancer and infectious diseases.”
BioNTech is also working on expanding its infectious diseases portfolio beyond COVID-19 with continued investments in influenza and cancer, with the BNT111 Melanoma mRNA immunotherapy candidate.
Recce Pharmaceuticals Limited today announced it had raised a total of A$12.4 million through a recently completed institutional placement.
On July 15, 2024, the US Department of Defense awarded the Company a grant for US$2 million.
As of August 5, 2024, the Company's pro forma cash position was A$19.8 million.
The Company confirmed it will advance clinical trials for intravenous use of developing a new class of synthetic anti-infective RECCE® 327 (R327) and topical applications of R327G, including Phase III clinical activities in Indonesia and IND-enabling activities.
RECCE® 327 is an intravenous and topical therapy being developed for the treatment of severe and potentially life-threatening infections caused by both Gram-positive and Gram-negative bacteria, including their superbug forms, such as Urinary Tract Infections, which are common infectious diseases caused by pathogens, such as Escherichia coli.
According to the World Health Organization, RECCE® 327 was added to the List of Antibacterial Products in Clinical Development in June 2024. It is the only compound classified as an adenosine triphosphate production disruptor.
As of April 2024, the Company was producing 5,000 GMP doses of RECCE® 327 per week. However, this product is not market-approved for human use.
Visiting areas where Valley Fever is common can put any mammal at risk of infection from breathing in fungal spores. Over the past decade, Valley Fever fungus (coccidioidomycosis) has sickened thousands of healthy people.
This fungus is found in regions like the southwestern United States, Mexico, and Central and South America.
In 2022, U.S. states reported a total of 17,612 cases of Valley Fever to the Centers for Disease Control and Prevention.
To address this health risk, Anivive Lifesciences Inc. recently announced that the National Institute of Allergy and Infectious Diseases (NIAID has awarded (#75N93024C00009) worth up to $33M to support the development of a Valley Fever preventive vaccine.
The funding will address enabling activities, including additional manufacturing, formulation, extensive safety testing, and an IND submission, before completing a human Phase 1 clinical trial.
"Anivive is honored to receive this NIAID contract, which will greatly accelerate our efforts to commercialize a vaccine to protect people against Valley Fever," said Dr. Edward Robb, Anivive Lifesciences Chief Strategy Officer and Principal Investigator, in a press release on August 2, 2024.
"This collaborative effort has delivered a significant step forward in the field of vaccinology and holds the potential to be the first vaccine to prevent a serious systemic fungal infection common to humans and animals," said Robb.
As of August 5, 2024, details of the phase 1 study were pending.
Additionally, this program is supported by Valley Fever Center for Excellence at the University of Arizona College of Medicine for the non-clinical development, Recipharm for the contract manufacturing, Quigley BioPharma for vaccine development support, and Latham BioPharm Group, part of Sia Partners for the program management, financial compliance, quality assurance, and additional technical subject matter expertise.

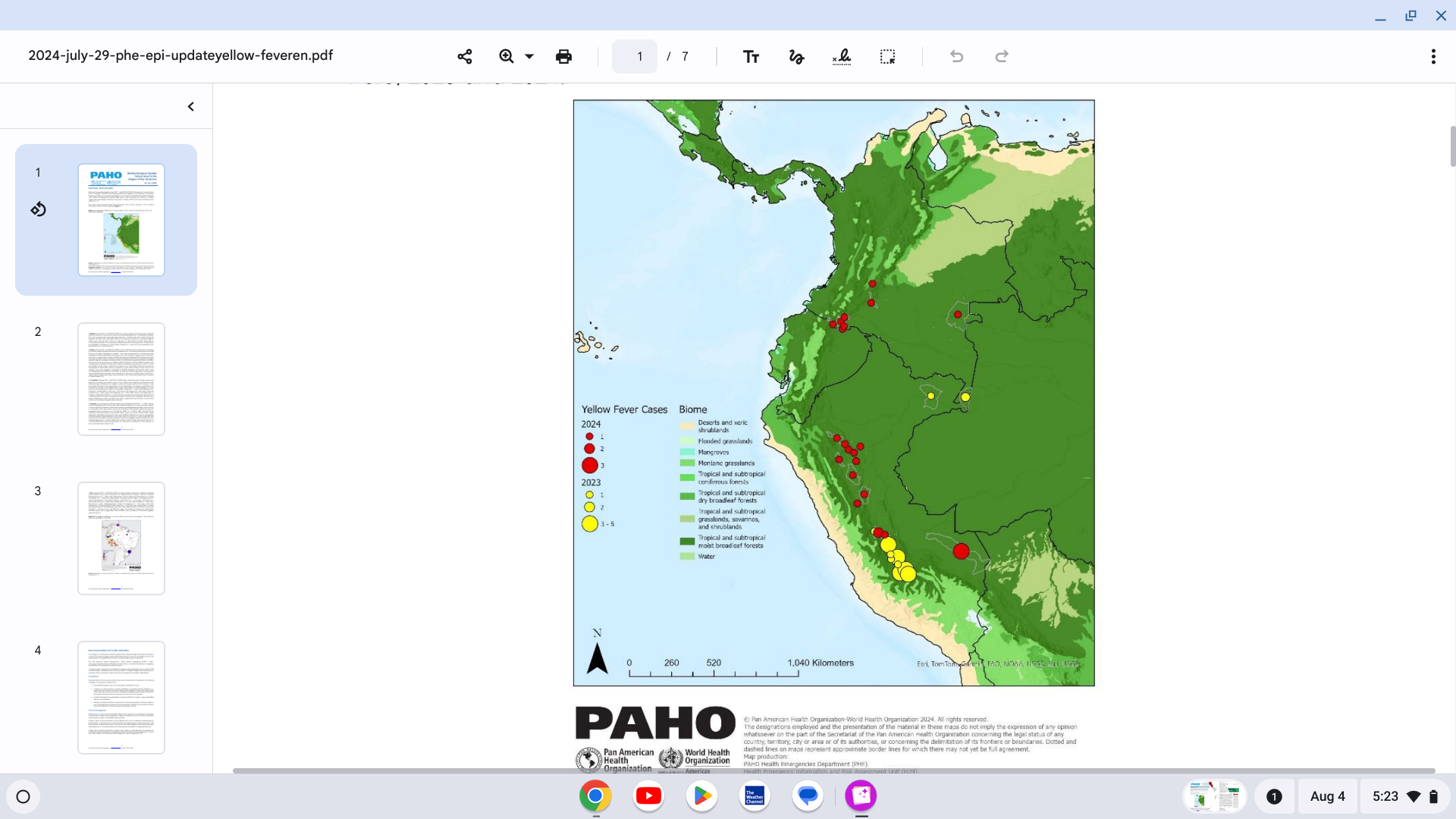
The Pan American Health Organization (PAHO) says countries in the Region of the Americas need to ensure that yellow fever vaccination coverage is uniformly greater than or equal to 95% to curtail future outbreaks.
Unfortunately, 33 confirmed cases of yellow fever, including 17 deaths, have been reported in the Americas throughout 2024. These cases have been reported in five countries in the Region of the Americas: the Plurinational State of Bolivia, Brazil, Colombia, Guyana, and Peru.
The PAHO confirmed on July 29, 2024, that these countries need to ensure that health authorities maintain routine vaccination and, at the same time, respond to possible outbreaks, such as in Columbia's mountain region.
Eight confirmed cases of yellow fever, including five deaths, have been reported in Columbia this year. One case had a history of vaccination against yellow fever.
From a local perspective, the Secretary of Health of Huila, Colombia, issued a Red Alert for rising numbers of confirmed yellow fever cases on August 2, 2024.
The U.S. Embassy says travelers planning to travel to this area of Colombia are encouraged to talk to their medical provider about proper precautions against this mosquito transmission and to prevent infection, such as vaccination.
As of August 2024, approved yellow fever vaccines are offered in various countries. In the United States, the YF-Vax vaccine is available at certified travel clinics and pharmacies.
The U.S. CDC recommends yellow fever vaccination for persons over nine months traveling to or living in areas of South America and Africa at risk of yellow fever. Because serious adverse events occur, clinicians should vaccinate only persons at risk for exposure to the yellow fever virus or require proof of vaccination for country entry.
The Africa Centers for Disease Control and Prevention (Africa CDC) has recently confirmed a 160% increase in mpox cases in Africa this year.
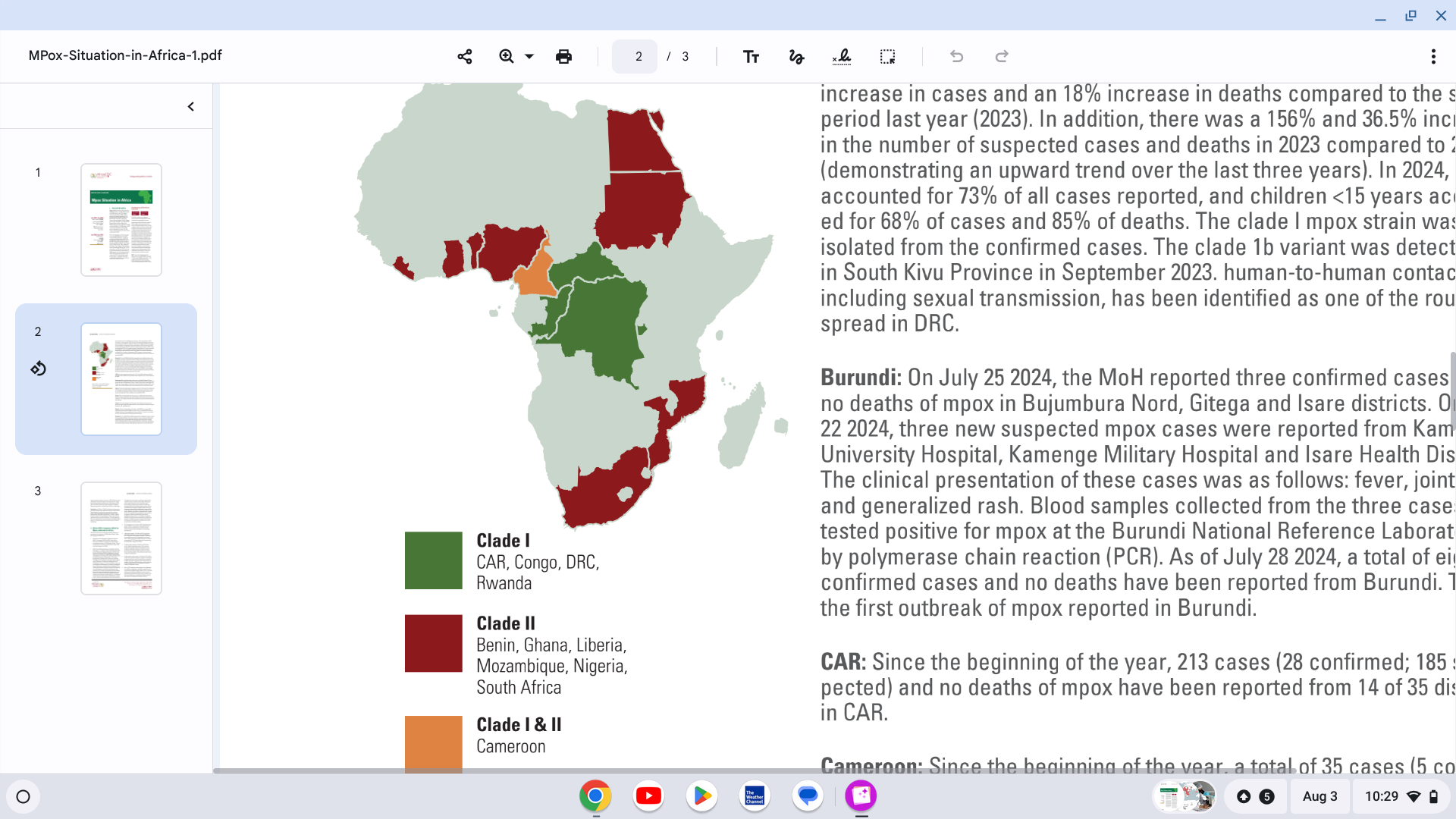
As of July 28, 2024, a total of 14,250 cases and 456 deaths (case fatality rate 3.2%) have been recorded in 10 African nations.
While the Democratic Republic of the Congo has reported the most cases in 2024, several other African countries, including Kenya, Cote d’Ivoire, and the Central African Republic, have reported new mpox outbreaks.
"While mpox is moderately transmissible and usually self-limiting, the case fatality rate has been much higher on the African continent compared to the rest of the world,” the Africa CDC wrote.
On June 27, 2024, the U.S. CDC provided a Mpox Update: Clinical Management and Outbreaks. The CDC recommends that people at high risk for this sexually transmitted disease should speak with a healthcare provider about vaccination options.
Since May 2022, the CDC has endorsed the use of Bavarian Nordic's JYNNEOS® (MVA-BN®, IMVAMUNE®, IMVANEX®) two-dose, mpox, and smallpox vaccine, which remains available at clinics and pharmacies in the U.S.
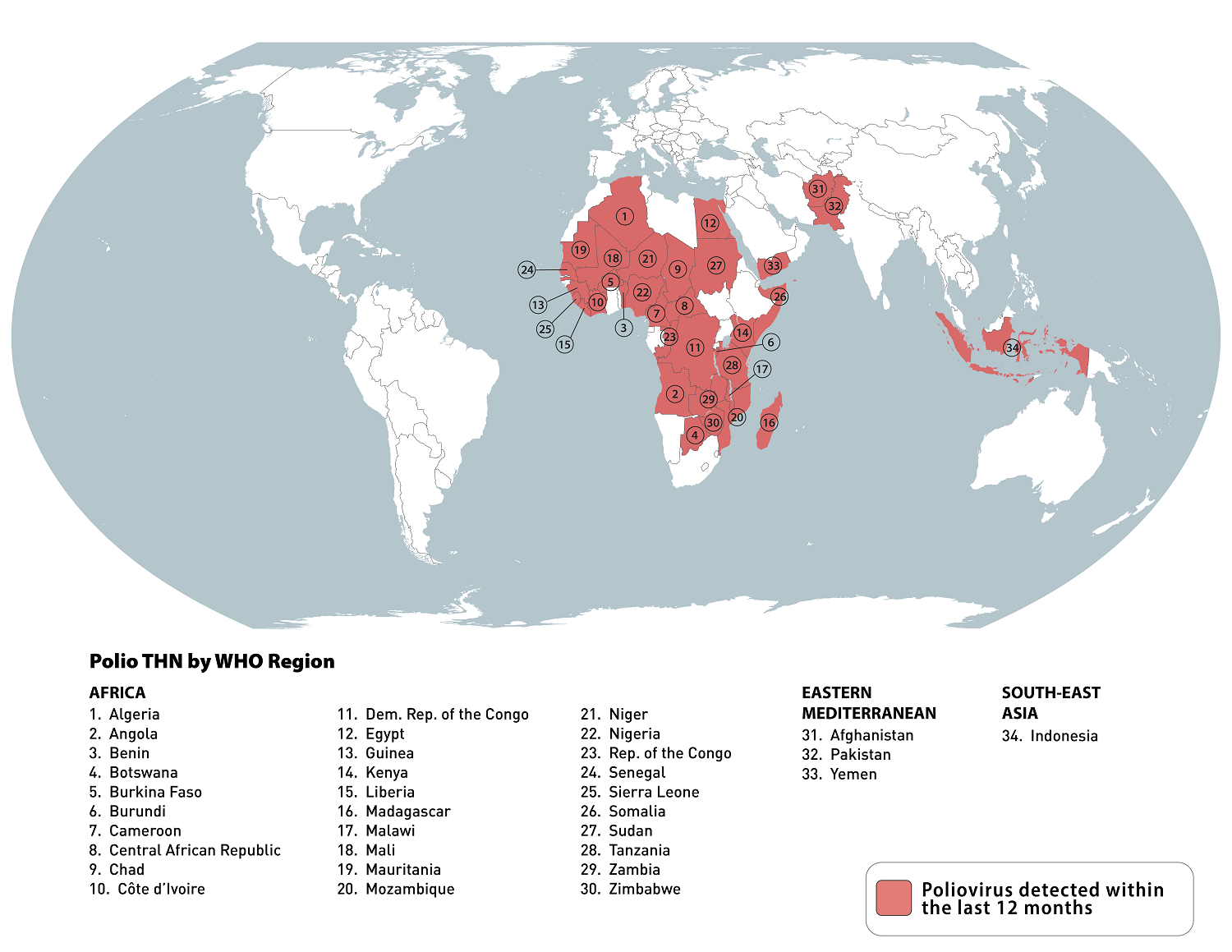
To eradicate polio, the Global Polio Eradication Initiative brings together the latest scientific knowledge on this vaccine-preventable disease. In late July 2024, the GPEI launched Team End Polio, a new campaign to unite fans everywhere around a common goal: to end polio for good.
Team End Polio is bringing together a world-class roster of athletes, global leaders, and advocates to raise awareness for polio eradication online and on the field.
This effect is essential since the risk of the emergence of a circulating vaccine-derived poliovirus type 1 (cVDPV1), Wild poliovirus type 1 (WPV1), or circulating vaccine-derived poliovirus type 3 (cVDPV3) has increased due to low vaccination coverage.
The GPEI also tracks the status of the poliovirus spreading every week. During the week of July 31, 2024, the following poliovirus reports were confirmed:
Pakistan: 11 WPV1-positive environmental samples
Chad: one cVDPV2 case and one positive environmental sample
Guinea: one cVDPV2 case
Liberia: two cVDPV2 positive environmental samples
Niger: three cVDPV2 positive environmental samples
Nigeria: six cVDPV2 cases and three positive environmental samples
Palestinian Territory: six cVDPV2 positive environmental samples
Sierra Leone: one cVDPV2 positive environmental sample
Yemen: one cVDPV2 case
The U.S. CDC's latest Travel Health Advisory says that before visiting any of 34 destinations, adults who have previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of the polio vaccine. Polio vaccines are offered at health clinics and pharmacies in the U.S.