Search API
The World Health Organization (WHO) recently reported that measles outbreaks continue in various countries in 2024, such as India.
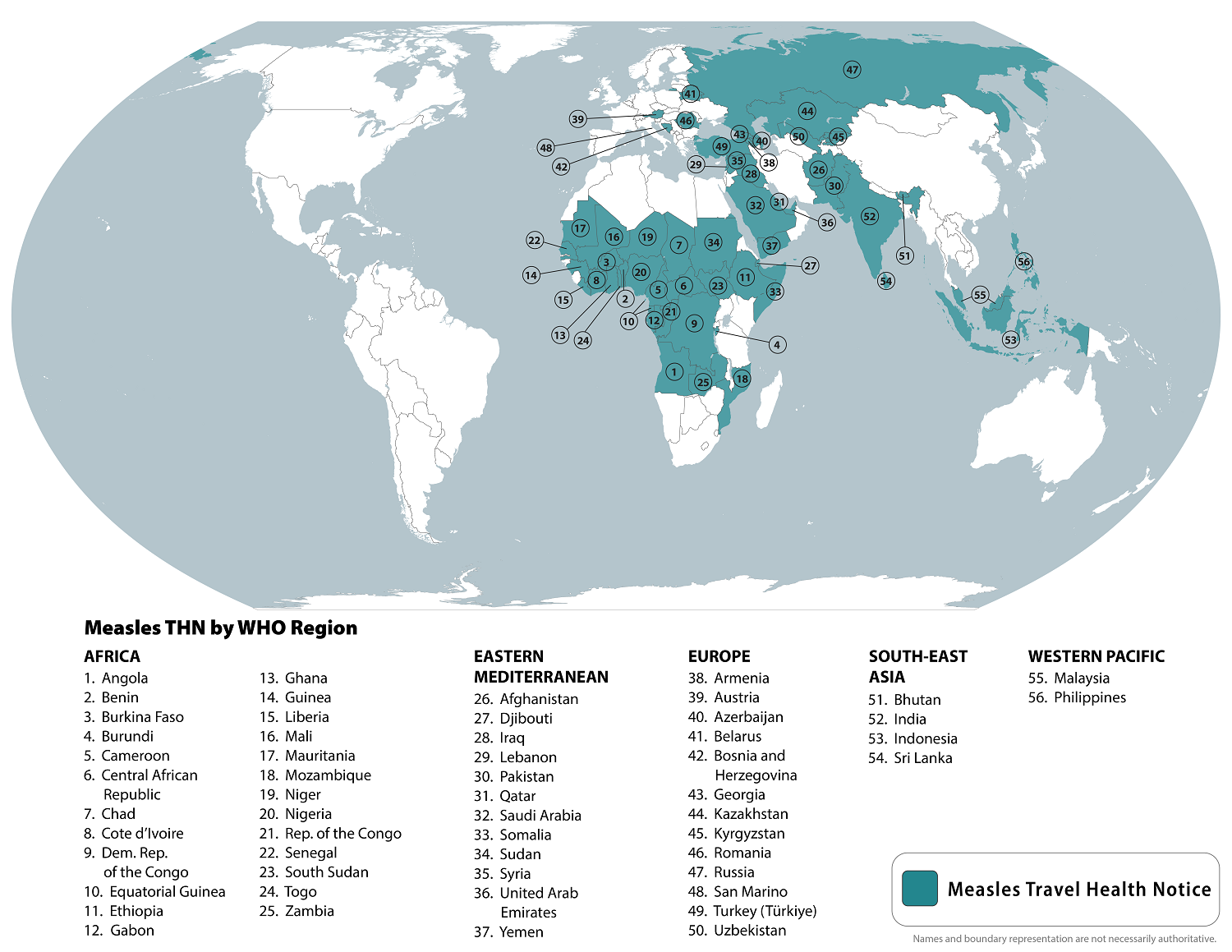
To alert international travelers, the U.S. Centers for Disease Control and Prevention (CDC) republished a global Watch-Level 1, Practice Usual Precautions, Travel Health Notice on August 14, 2024, identifying measles outbreaks in 56 countries.
Within the United States, the CDC has reported 219 measles cases in 27 jurisdictions this year, led by the states of Illinois and Oregon. Most of these cases are related to unvaccinated travelers.
The CDC writes that 'all international travelers should be fully vaccinated against measles with two doses of the measles-mumps-rubella (MMR) vaccine, including an early dose for infants 6–11 months.
However, the CDC does not recommend a third dose of MMR vaccination during or when visiting measles outbreaks.
In the U.S., MMR vaccines are available at most clinics and community pharmacies.
The World Health Organization (WHO) recently announced that mass mpox vaccination is not recommended against this sexually transmitted disease.
On August 17, 2024, the WHO confirmed international travelers who may be at risk based on an individual assessment with their healthcare provider may wish to consider vaccination before visiting countries reporting mpox outbreaks, such as in Africa.
Recently, the WHO Director-General announced that he had triggered the process for Emergency Use Listing of mpox vaccines. The WHO currently recommends two new vaccines against mpox disease and continues listing an older smallpox vaccine as an option.
The United States Food and Drug Administration (FDA) Approved Bavarian Nordic's JYNNEOS® (MVA-BN, IMVANEX®, IMVAMUNE®) Smallpox and Mpox Vaccine on September 24, 2019. The U.S. began offering the JYNNEOS vaccine to healthcare staff in Boston on May 24, 2022, in response to the Clade 2 mpox global outbreak.
JYNNEOS remains available in the U.S. at certain clinics and pharmacies.
Additionally, Japan's K.M. Biologics' LC16 "KMB" freeze-dried smallpox vaccine has been approved by the WHO, Japan's Ministry of Health, Labor, and Welfare, and other countries.
The older ACAM2000® live vaccinia virus vaccine is authorized to prevent mpox and smallpox infections in various countries. However, the safety profile of ACAM2000 vaccination includes risks for myocarditis and pericarditis.
The WHO writes, 'Results from vaccine effectiveness studies indicate that a good level of protection is provided against mpox (Clade 2) following vaccination. Further studies on the use of vaccines for mpox (Clade 1) will provide additional information on the effectiveness of these vaccines in different settings.'
The Centers for Disease Control and Prevention (CDC) today issued a Health Alert Network Health Advisory (CDCHAN-00515) to notify clinicians and public health authorities of an increase in Oropouche virus disease cases confirmed in the Americas region.
The CDC issued this alert because the initial clinical presentation of the Oropouche virus may confuse providers as the symptoms are similar to those of dengue, Zika, and chikungunya.
Between January and August 1, 2024, more than 8,000 cases of Oropouche virus disease were reported, including two deaths and five cases of vertical transmission associated with fetal death or congenital abnormalities.
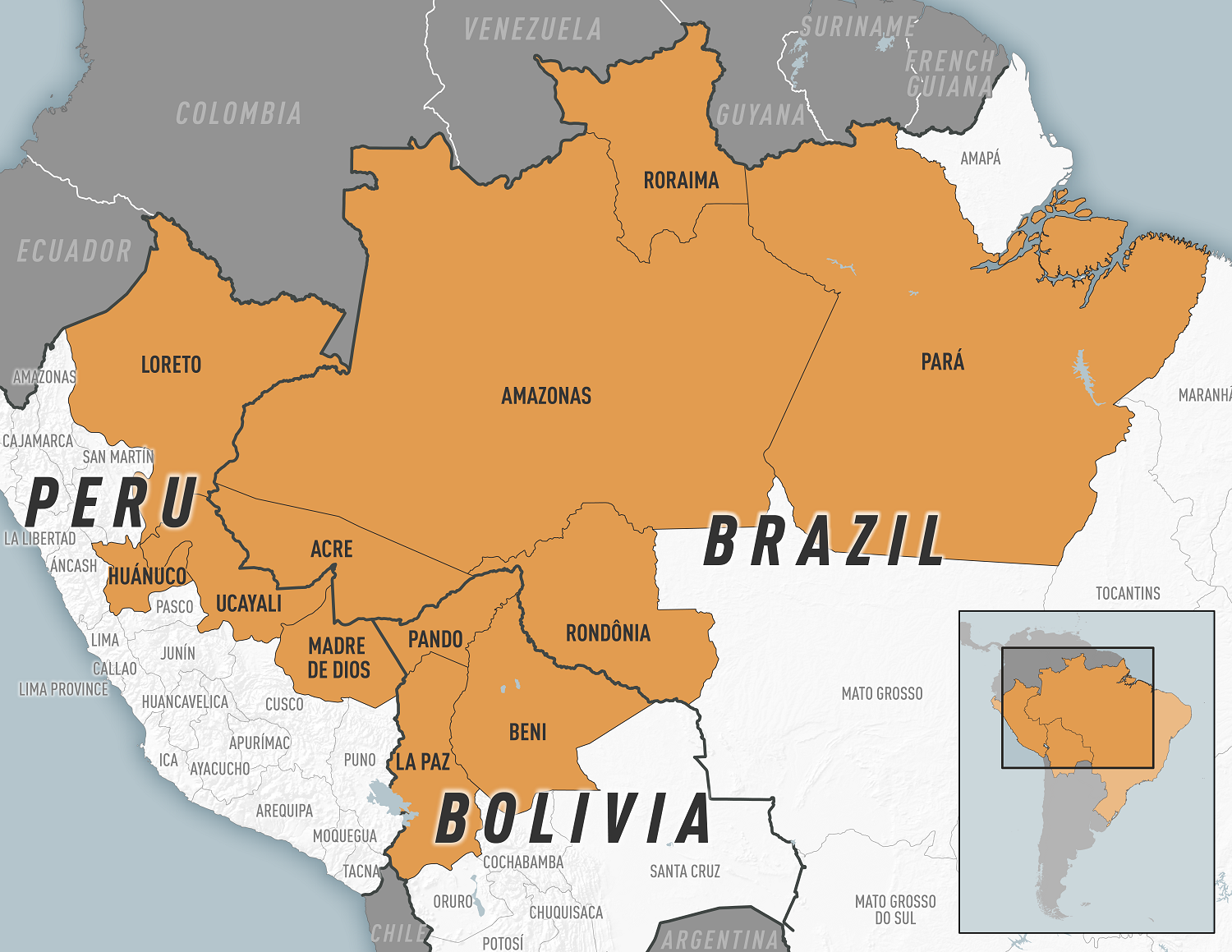
These Oropouche cases originate from endemic areas in the Amazon basin and regions in South America and the Caribbean.
The virus was first detected in 1955 in Trinidad and Tobago and is endemic in the Amazon basin.
As of August 16, 2024, Brazil, Bolivia, Peru, Colombia, and Cuba were among the countries reporting cases. As testing and surveillance for Oropouche virus disease increase in the Americas, reports of cases from additional countries are expected.
Throughout 2024, travel-associated cases have been identified in travelers returning to the United States and Europe from Cuba and Brazil.
This CDC Health Advisory offers advice on evaluating and testing travelers who have been in impacted areas with signs and symptoms consistent with Oropouche virus infection.
It also raises awareness of the possible risk of vertical transmission (e.g., from gestational parent to fetus during pregnancy) and associated adverse effects on pregnancy.
The CDC issued a Level 2 Travel Health Notice in August 2024, suggesting pregnant women reconsider non-essential travel to areas with Oropouche virus outbreaks, such as Brazil and Cuba.
The new alert also highlights prevention measures to mitigate the additional spread of the virus and potential importation into unaffected areas, including the U.S.
Oropouche virus belongs to the Simbu serogroup of the genus Orthobunyavirus in the Peribunyaviridae family.
According to the CDC, approximately 60% of people infected with the Oropouche virus become symptomatic. The incubation period is typically 3–10 days. Providers should contact state, tribal, local, or territorial health departments to facilitate diagnostic testing.
As of August 2024, there are no approved Oropouche vaccines available.
According to local news reporting, an official with the health ministry said that Pakistanis traveling through Iran are being screened for dengue fever.
“Health stations have been established in Mirjaveh and Rimdan borders, in Sistan-Baluchestan Province, to screen Pakistanis to prevent the entry of travel-associated infectious diseases to the country,” ISNA quoted Jafar Miadfar, head of Iran’s Emergency Organization.
These travelers are checked for clinical symptoms such as fever, chills, low consciousness, and skin rashes. Also, needed laboratory tests are implemented, and infected individuals will be prevented from entering Iran, the official added.
The Tehran Times reported on August 16, 2024, that thanks to effective border control measures and quality healthcare, the number of people entering the country with dengue fever has significantly decreased compared to the previous months.
In Europe, Aedes albopictus mosquitos transmit the virus to people.
While dengue screening is a new aspect of international travel, it is related to the increasing number of confirmed cases in Europe.
Dengue epidemics were first reported in the Eastern Mediterranean Region in 1998.
On August 16, 2024, the European Centre for Disease Prevention and Control (ECDC) confirmed local autochthonous (local) dengue cases in Europe this year.
The first dengue case that presented symptoms was in June in France. Overall, France reported six locally acquired dengue cases in 2024.
Previously, the ECDC reported locally acquired dengue virus outbreaks in Italy, Spain, and Croatia, as well as in Djibouti, Egypt, Oman, Pakistan, Saudi Arabia, Somalia, Sudan, and Yemen.
As of August 2024, the World Health Organization (WHO) does not endorse dengue virus testing for international travelers.
However, the WHO does recommend dengue vaccination one month before visiting outbreak areas, such as the Region of the Americas.
The European Centre for Disease Prevention and Control (ECDC) today announced the likelihood of infection with mpox virus (MPXV) clade I for EU/EEA citizens traveling to or living in the affected areas and having close contact with affected communities is high, while the likelihood of infection is low when contacts with affected communities are avoided.
The severity of the mpox disease is expected to be low.
Overall, the ECDC's updated risk assessment for these populations is moderate and low, respectively.
“As a result of the rapid spread of this (mpox) outbreak in Africa, ECDC has increased the level of risk for the general population in the EU/EEA and travelers to affected areas. Due to the close links between Europe and Africa, we must be prepared for more imported clade I cases,” says Pamela Rendi-Wagner, Director of ECDC, in a press release.
As of August 16, 2024, the ECDC says to contain any possible clade 1 mpox outbreak in Europe, detecting cases and preventing secondary transmission is vital. This goal can be achieved through:
- Providing advice to travelers to affected areas on national guidance for vaccination against mpox before traveling. In many countries, the JYNNEOS® (MVA-BN®) vaccine is available. And offering travel advice to people visiting or returning from African countries with confirmed MPXV clade I outbreaks.
- Rapidly isolating suspected cases until proven negative and, if positive, until symptom resolution. And implementing contact tracing and testing close contacts of confirmed cases following ECDC testing protocols.
- Continuing risk communication activities and working with civil society organizations to engage population groups at higher risk of infection.
On August 14, 2024, the WHO Director-General determined that the increase of mpox clade 1 virus cases in African countries constitutes a new public health emergency.
In the United States, no clade 1 mpox cases have been detected by the U.S. CDC, and the JYNNEOS vaccine remains available.

BioNTech SE today announced top-line results from a Phase 3 clinical trial evaluating a mRNA influenza and COVID-19 vaccine candidate in healthy adults.
The combination vaccine comprises Pfizer’s mRNA-based influenza vaccine candidate with the companies’ licensed COVID-19 vaccine.
The Phase 3 trial measured two primary immunogenicity objectives (immunogenicity against the SARS-CoV-2 coronavirus and immunogenicity against influenza A and B), of which one objective was met.
The companies are evaluating adjustments to the combination vaccine candidate to improve immune responses against influenza B and will discuss next steps with health authorities.
“We are dedicated to developing combination vaccines that provide broader protection against multiple respiratory diseases,” said Prof. Ugur Sahin, M.D., CEO and Co-founder of BioNTech, in a press release on August 16, 2024.
“The insights gained from this combination vaccine trial are highly valuable and will play a crucial role in guiding the further development of Pfizer’s and our combination vaccine program against influenza and COVID-19."
"We are committed to drawing on our experience in developing mRNA-based vaccine candidates against multiple antigens and believe we can successfully accomplish this task in collaboration with our partner Pfizer.”
As of August, updated influenza vaccines for the 2024-2025 flu season in the United States became available at pharmacies.

The National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health announced topline results from a preliminary PALM 007 phase 2 clinical trial analysis.
The NIAID reported on August 15, 2024, that the study did not meet its primary endpoint of a statistically significant improvement in time to lesion resolution within 28 days post-randomization for patients with mpox who were administered SIGA’s oral tecovirimat (TPOXX®) a highly targeted antiviral treatment, versus placebo.
However, a meaningful improvement was observed in patients receiving tecovirimat whose symptoms began seven days or fewer before randomization and those with severe or more significant disease.
This study's finding is essential given Africa's ongoing clade 1 mpox virus outbreak.
“These data showing maximum benefit in patients treated early and with severe disease are entirely consistent with the mechanism of action of tecovirimat and with the studies in animals that led to U.S. FDA approval of this medicine for smallpox..... We believe these data warrant further investigation and support our view that post-exposure prophylaxis will be vital for the treatment of severe cases of mpox and all cases of smallpox,” stated Dennis Hruby, Chief Scientific Officer, in a press release.
On July 22, 2024, the U.S. government exercised a procurement option to deliver approximately $113 million in TPOXX treatment courses.
TPOXX is currently available in the U.S., United Kingdom, Canada, and Europe.

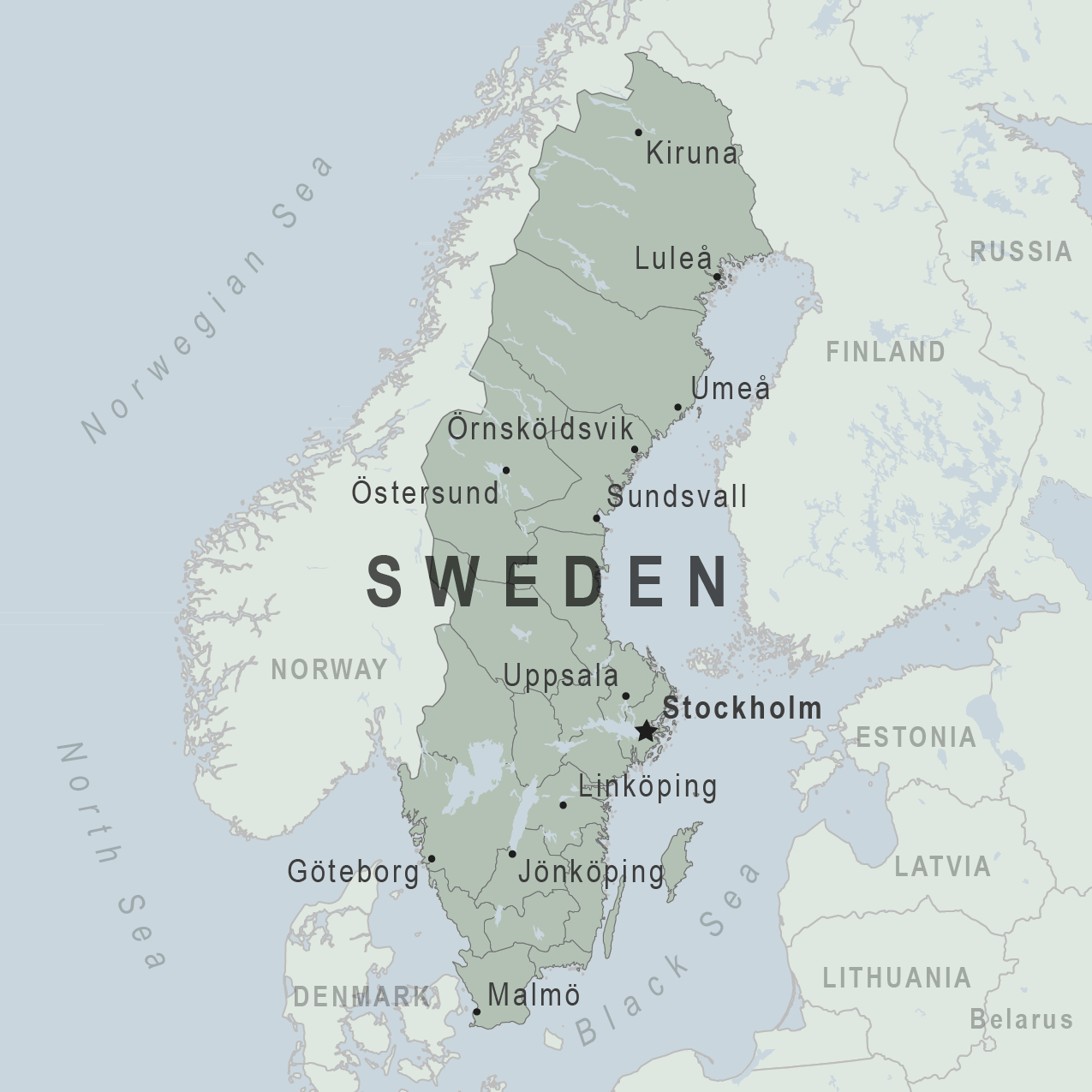
The Public Health Agency of Sweden today announced a person who sought care at Region Stockholm had been diagnosed with mpox caused by the clade I variant.
According to the agency, on August 15, 2024, this person received care and rules of conduct, says Magnus Gisslén, state epidemiologist at the Public Health Agency of Sweden, in a press release.
The agency says this is the first case caused by the sexually-transmitted clade I virus to be diagnosed outside the African continent. The person was infected during a recent stay in that part of Africa.
Recently, the World Health Organization declared the clade I mpox outbreak in the Democratic Republic of Congo and neighboring countries a public health emergency of international concern.
The fact that a patient with mpox is treated in the country does not affect the risk to the general population, a risk that the European Centre for Disease Prevention and Control currently considers very low. A new assessment is expected shortly.
However, occasional imported cases like the current one may continue to occur.
In the United States, the Clade 2 mpox outbreak began in May 2022 and has substantially diminished in 2024. However, the JYNNEOS smallpox / mpox vaccine continues to be available at specific clinics and pharmacies in the U.S.